GMP-Grade MaxNuclease™
Catalog #GMP-NUC-SE101
Degrades all DNA & RNA for biologics manufacturing

About MaxNuclease™
What is MaxNuclease?
Non-Specific Endonuclease from Serratia marcescens
MaxNuclease is identified from Serratia marcescens and is genetically engineered and expressed in E. coli under cGMP manufacturing standards. MaxNuclease is a non-specific nuclease with high activity and specificity that degrades all forms of nucleic acids including single- and double-stranded, linear and circular nucleic acids.
What does MaxNuclease do?
DNA/RNA Degradation into 2-5 base oligonucleotides
MaxNuclease is a homodimer of two 30 kDa subunits containing two disulfide bonds that are essential for activity and stability. It hydrolyzes internal phosphodiester bonds between nucleotides in nucleic acids to produce 5'-monophosphate oligonucleotides of 2-5 bases in length.
What is MaxNuclease used for?
High-Efficiency Endonuclease for Biologics Manufacturing
Products such as lentiviral or AAV vectors, antibody drugs, vaccines, and recombinant protein drugs are expressed and produced by continuously passaged cells. Even after a fine purification process, host nucleic acids may remain in the products, and the residual nucleic acids may cause pathogenicity, tumorigenesis, etc. Residual nucleic acids need to be removed to a safe level in the final drug product.
Product Features
Strict quality management for clinical manufacturing
FDA Drug Master Files: DMF #036799
Ordering Information
MaxNuclease is manufactured according to cGMP guidelines and is available in long-term bulk supply with batch-to-batch consistency to ensure suitability for industrial applications.
Order Now
Order GMP-Grade MaxNuclease online via the product page. To pay via PO, please send your order information to orders@kactusbio.us.
Request a Test Sample
To request a test sample of MaxNuclease, please click below to fill our our sample request form.
Product Specifications
Contact us: support@kactusbio.us.
Quality Control Criteria
Product Performance Validation
Host Cell DNA Removal
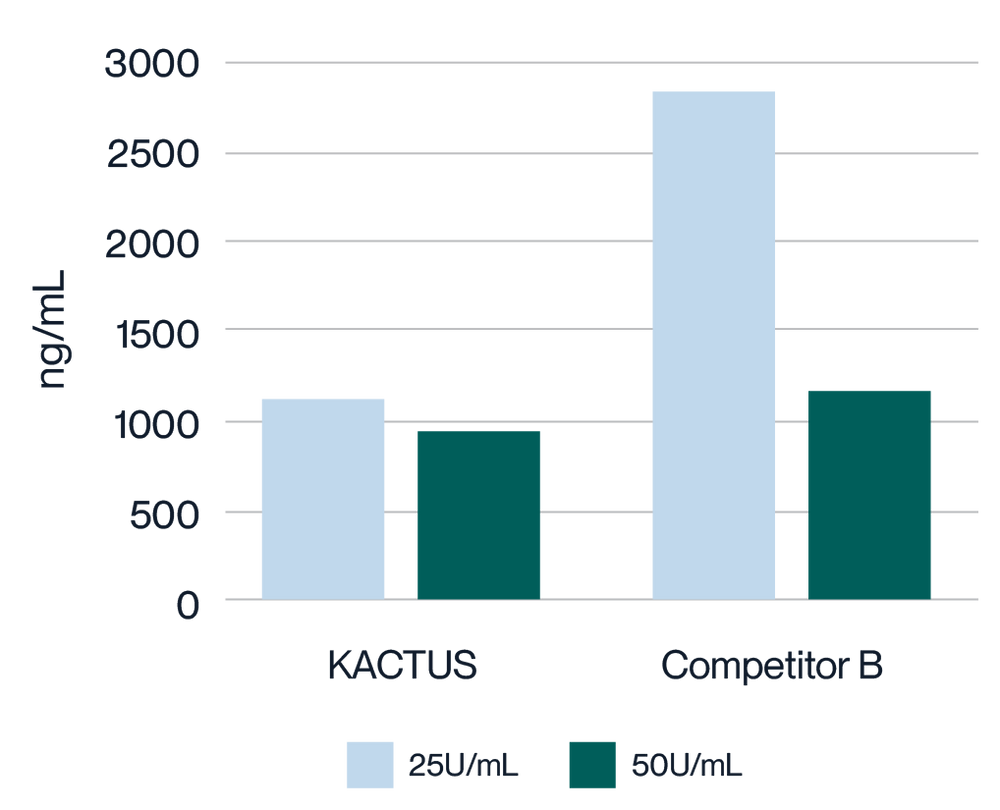
Virus harvest solution was treated with 25U/mL and 50U/mL endonuclease at 37°C for 2 hours, respectively. Detection of Host Cell DNA (HCD) residue was analyzed. KACTUS MaxNuclease has higher degradation activity versus Competitor B demonstrated by lower HCD residue for both 25U/mL and 50U/mL working concentrations.
Plasmid DNA Removal
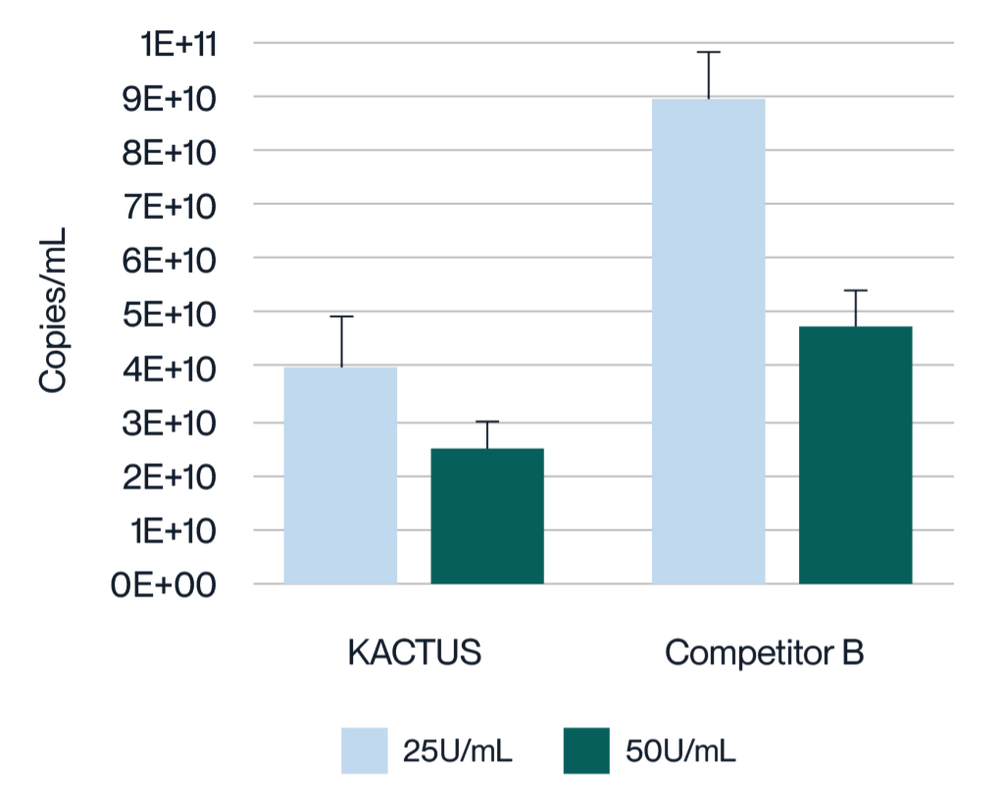
Virus harvest solution was treated with 25U/mL and 50U/mL endonuclease at 37°C for 2 hours, respectively. Detection of plasmid DNA (pDNA) residue was analyzed. KACTUS MaxNuclease has higher degradation activity versus Competitor B demonstrated by lower pDNA residue for both 25U/mL and 50U/mL working concentrations.
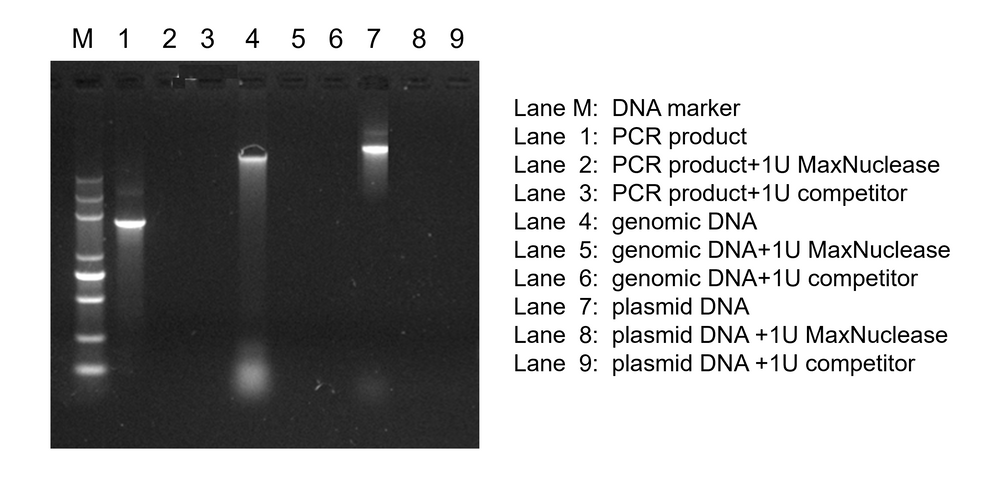
Degradation of PCR Product, Genomic DNA, and Plasmid DNA
MaxNuclease added to PCR product, genomic DNA, and plasmid DNA shows comparable degradation activity of nucleic acids to leading competitors.
Practical Applications of MaxNuclease™
MaxNuclease is identified from Serratia marcescens and is genetically engineered and expressed in E. coli under cGMP manufacturing standards. MaxNuclease is a non-specific nuclease with high activity and specificity that degrades all forms of nucleic acids including single- and double-stranded, linear and circular nucleic acids.
Our GMP Compliance
KACTUS MaxNuclease has been developed and verified with reference to cGMP production standards. The manufacturing process is in accordance with GMP production standards to ensure the traceability of raw materials in the production process. At present, this product has also been filed with the U.S. FDA Drug Master Files (DMF #036799).
Quality Management System
→ ISO13485 Accreditation
→ Digital Manufacturing Execution System (MES)
→ Process and Analytical method validation
→ Batch-to-batch stability and consistency
→ Free from antibiotic residues and raw materials of animal origin
→ Comprehensive records for batch production
→ Pharmaceutical Class A & C Clean Room
→ Validated and maintained equipment
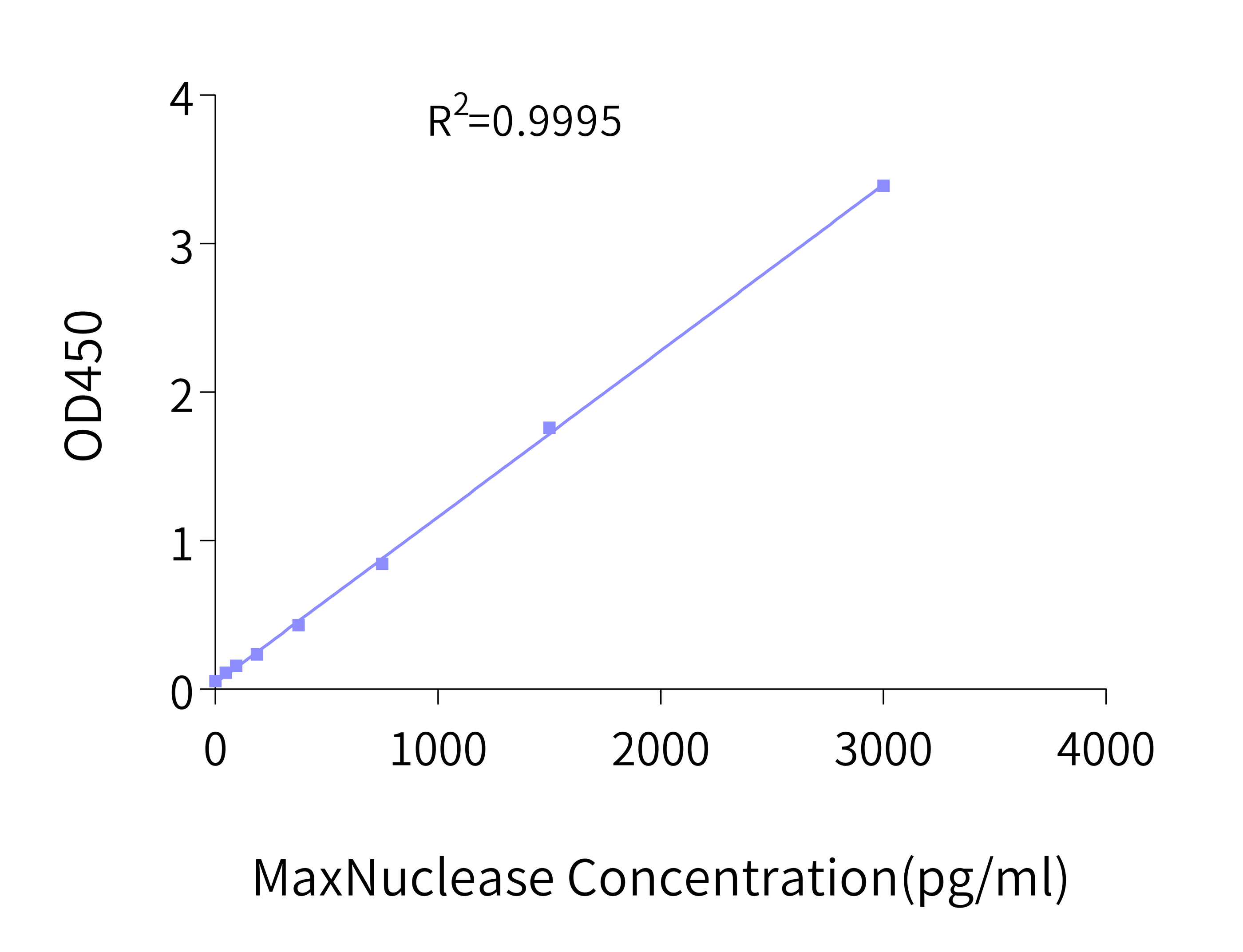
MaxNuclease ELISA Kit
After the nucleic acid is removed by MaxNuclease for biological products such as viral vectors and vaccines, it is also necessary to evaluate the residual MaxNuclease in the system. To this end, KACTUS has carefully developed a highly sensitive and specific residue detection kit, MaxNuclease ELISA Kit, with a sensitivity of up to 23 pg/mL.