After successful IND approval this past July, we are excited to announce the NK therapy NK510 — developed using KACTUS’ portfolio of proteins and enzymes for cell therapy — received clinical trial approval from the National Medical Products Administration (NMPA), paving the way for its use in treating advanced solid tumors. NK510 is an allogeneic, universal NK cell therapy developed with Base Therapeutics' proprietary base editing technology.
KACTUS, the strategic partner of Base Therapeutics, is the exclusive supplier of GMP-grade AccuBase™, the world’s first "off-the-shelf" cytosine base editor. As part of our range of gene editing enzymes for research, this key raw material is used in the clinical manufacturing of NK510. This milestone marks significant progress in NK cell therapy, and underscores the crucial role of AccuBase™ base editor in enabling the efficient and scalable production of innovative cell therapies. Moreover, it also demonstrates KACTUS’ strong manufacturing capability of GMP-grade enzymes with complex structures and delicate functions.
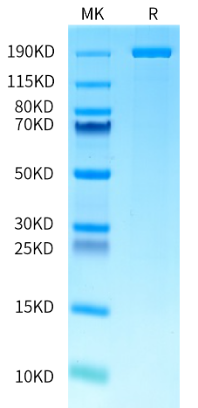
AccuBase™ is the world’s first, and currently the only, off-the-shelf, protein-based cytosine base editor (CBE) on the market. It was independently developed by Base Therapeutics with exclusive global IP rights. AccuBase™ can precisely convert cytosine (C) to thymine (T) with nearly zero off-target effects. This high-efficiency, precise base editing capability has been validated across various cell types, making it an invaluable tool in gene editing.
Key Features of AccuBase™
High-Efficiency Base Editing Activity: AccuBase™ effectively knocked out PD1, B2M, and TRAC proteins in primary T cells, with knockout efficiencies exceeding 80% and up to 96% in certain cases.
Near-Zero Off Target Efficiency: Genome-wide off target analysis demonstrated that AccuBase™ achieves off-target levels comparable to control groups, making it an extremely precise tool for gene editing.

No INDELs or Chromosomal Translocations: In contrast to Cas9 protein, AccuBase™ does not cause insertions and deletions (INDELs) or chromosomal translocations, enhancing the safety profile of gene editing applications.
In NK510, the GMP-grade AccuBase ™ enabled high-precision gene editing of NK cells, with an overall efficiency of more than 90% enhancing their ability to overcome cancer cell immune evasion, while still maintaining the effective target and killing cancer cells with an efficiency exceeding 90%. By allowing precise C-to-T conversions without causing DNA double-strand breaks, AccuBase™ ensured minimal off-target effects, enhancing the safety and effectiveness of NK510.
Manufacturing and Global Supply of AccuBase™ by KACTUS
By leveraging the exclusive Structure Aided Design and Multiplex Screening SAMS™ platform, KACTUS has successfully developed the process to stably produce AccuBase ™ with high stability, high purity, and high activity. The manufacturing of AccuBase™ is conducted in strict compliance with GMP standards, meeting the requirements of IND and BLA filing. Currently, Base Therapeutics has granted KACTUS exclusive rights for manufacturing and global sales of the AccuBase™.
KACTUS offers cost-effective, off-the-shelf Research-Grade and GMP-grade AccuBase™, both of which are in stock globally and ready to ship to the end user the next day. By providing an off-the-shelf base editor, we aim to fill the unmet needs in current gene editing therapy research, development and manufacturing.
About Base Therapeutics
Base Therapeutics was founded in April 2021, specializing in the research and development of novel gene-editing NK cell therapy and gene therapy products. They have developed the patented AccuBase base editing enzyme, which has global Freedom To Operate (FTO) and can achieve highly efficient gene editing both in vitro and in vivo with near-zero off-target effects. In addition, they have developed ePE, a highly efficient prime-editing technology. Based on these proprietary foundational technologies, Base Therapeutics focuses on developing First-in-Class BED-NK and BEAT-CAR-T product pipelines, as well as multiple in vivo base editing product lines.
About KACTUS
KACTUS is an innovation-driven company specializing in recombinant proteins and enzyme raw materials, dedicated to serving the development and production of biopharmaceuticals. Since its establishment in 2018, KACTUS has accumulated rich experience in protein R&D and production through its unique structure-designed protein development platform SAMS™. KACTUS has built its own GMP facility and certified quality system, providing various GMP-grade raw proteins and enzymes to meet the needs of cell and gene therapy drug development. Several GMP-grade products have completed FDA DMF filings.
For more technical information on our AccuBase™ base editor, access our brochure or visit the product page. To learn more about our gene editing product offerings, visit our Gene Editing Enzymes & Reagents page.