Background
The key to linear mRNA therapy lies in efficiently synthesizing high-quality mRNA in vitro and then delivering mRNA to the human body through a delivery system. The in vitro synthesis of mRNA cannot be done without high-quality raw material enzymes. KACTUS has a particular focus on the mRNA therapy market we've used our unique functional recombinant protein and high-activity enzyme production platform, SAMS™, to develop a full series of high-quality raw material enzymes required for in vitro synthesis of mRNA (GMP-Grade & GMP-Ready). Our GMP products have analytical method verification and undergo comprehensive quality release testing to meet the requirements for upstream raw material enzymes.
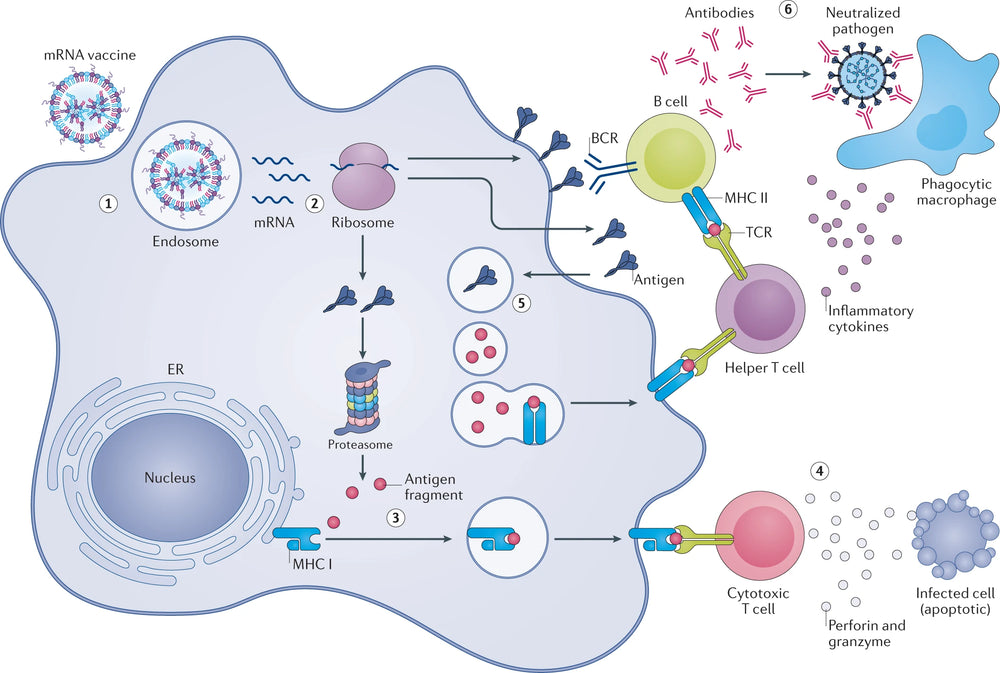
Figure 1. Mechanism of action of mRNA vaccines [1].
Enzymes for every step of RNA synthesis
Restriction Endonucleases for Plasmid Linearization
The template for mRNA in vitro transcription is typically plasmid DNA, and needs to be linearized before transcription. Linearization ensures the acquisition of mRNA transcripts of definite length and sequence. KACTUS provides restriction endonucleases such as BsaI, BspQI, SapI, XbaI, etc., for the preparation of linearized templates.
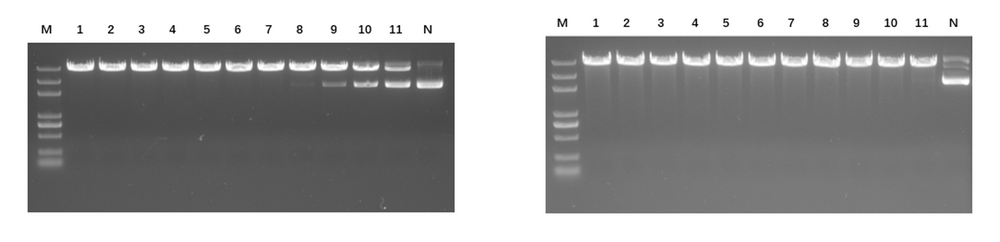
Figure 2. (Left) BsaI enzyme activity detection. 1μg of plasmid was added to a 20 μL system, along with 1 μL BsaI (well 1 is undiluted, wells 1-11 are serial 2-fold dilutions), and digested for 30 minutes. The enzyme activity is ≥20 kU/mL. (Right) BsaI star activity detection. 1 μg of plasmid is added to a 20 μL system, along with 1 μL BsaI (well 1 is undiluted; wells 1-11 are serial 2-fold dilutions) and digested for 16 hours. No star activity is observed.
In Vitro Transcription (IVT) Reagents
In Vitro Transcription (IVT) is a technique that generates mRNA by mimicking the internal transcription process enzymatically, using linearized plasmid DNA as a template.
Available Products:
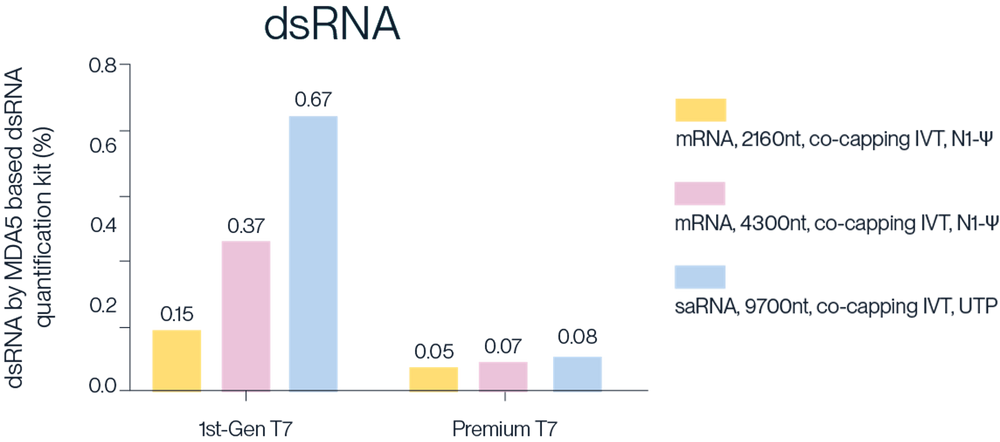
Figure 3. The first generation T7 and second generation Premium T7 were used for co-transcriptional capping on different templates to synthesize mRNA and saRNA of different lengths. After purification by lithium chloride precipitation, the dsRNA content were measured. The results show that Premium T7 RNA Polymerase can effectively reduce the production of dsRNA.
Note: MaxPure™ T7 RNA Polymerase is the commercial name for Premium T7 RNA Polymerase (RUO or GMP)
Enzymes for Capping & Tailing
The mRNA produced by IVT has a triphosphate group at the 5’ end, which has strong immunogenicity, necessitating capping and tailing modifications in industrial production. KACTUS offers Vaccinia Capping Enzyme and mRNA Cap 2´-O-Methyltransferase needed for capping modifications, as well as E.coli Poly(A) Polymerase required for tailing modifications. To precisely quantify important indicators such as "capping rate" and "Poly(A) tail product length," we have carefully established an LC-MS platform and developed mature mass spectrometry detection methods to control the release of enzyme products by batch. We can also provide capping rate and tailing determination services according to your needs.
Available Products:
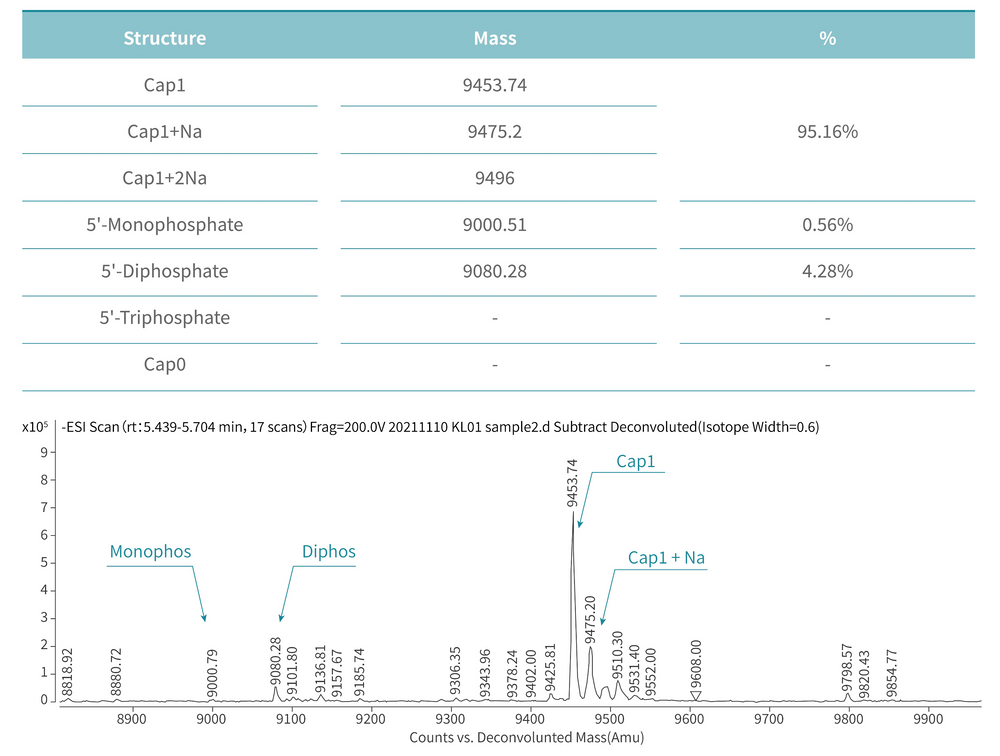
Figure 4. mRNA is capped with Cap1 structure using Vaccinia Capping Enzyme and mRNA Cap 2´-O-Methyltransferase from KACTUS. The capping rate is 95.16% detected via our LC-MS platform. (Note: The capping rate is related to the performance of the enzyme and factors such as the secondary structure of mRNA.)
Enzymes for circRNA In Vitro Synthesis
Linear mRNA obtained from IVT can be further circularized to obtain circRNA. The circularization methods include Type I intron self-splicing, Type II intron self-splicing, T4 RNA Ligase, etc. KACTUS provides T4 RNA Ligase I, T4 RNA Ligase II, and RNase R for in vitro RNA circularization and purification processing of circRNA.
Available Products:
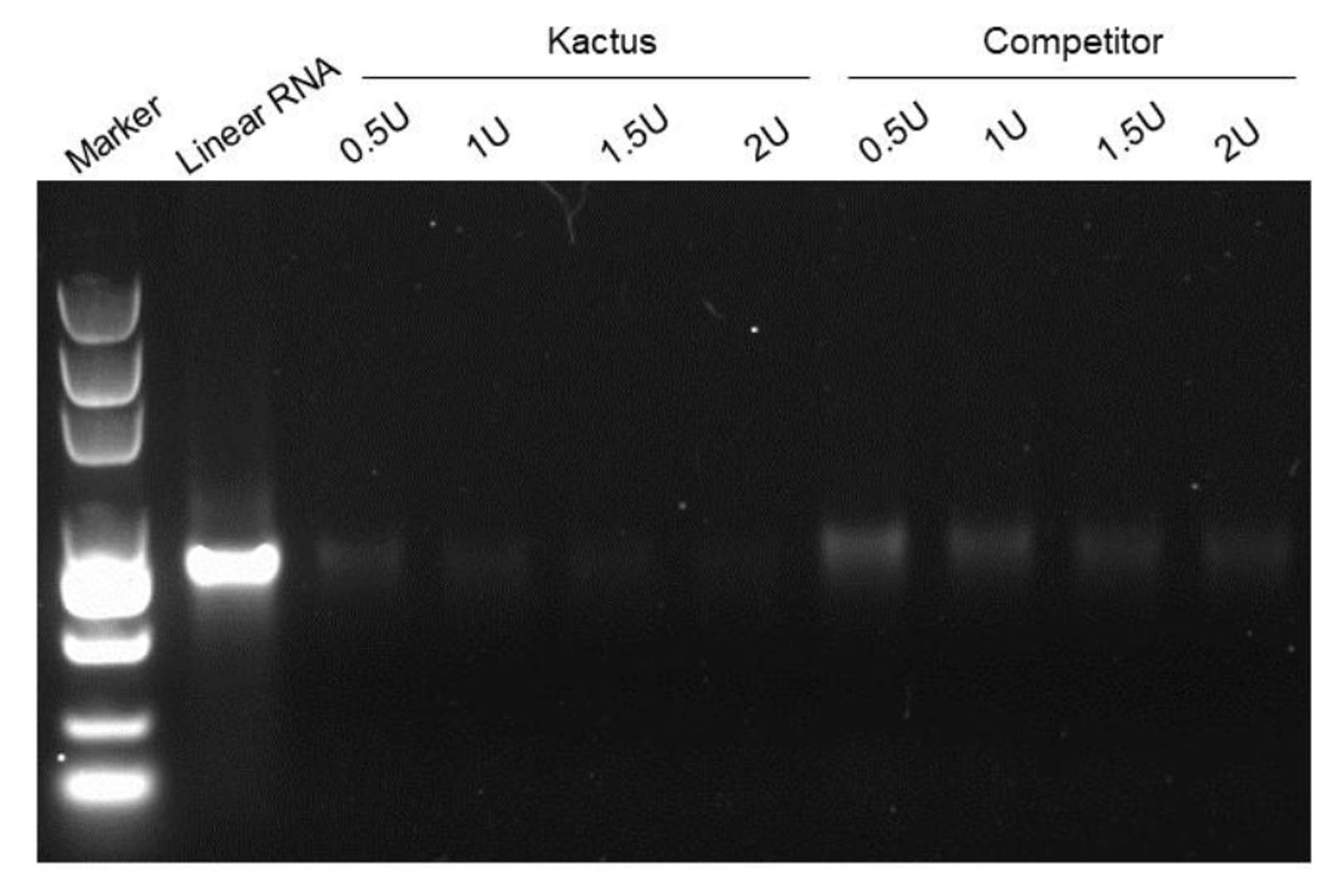
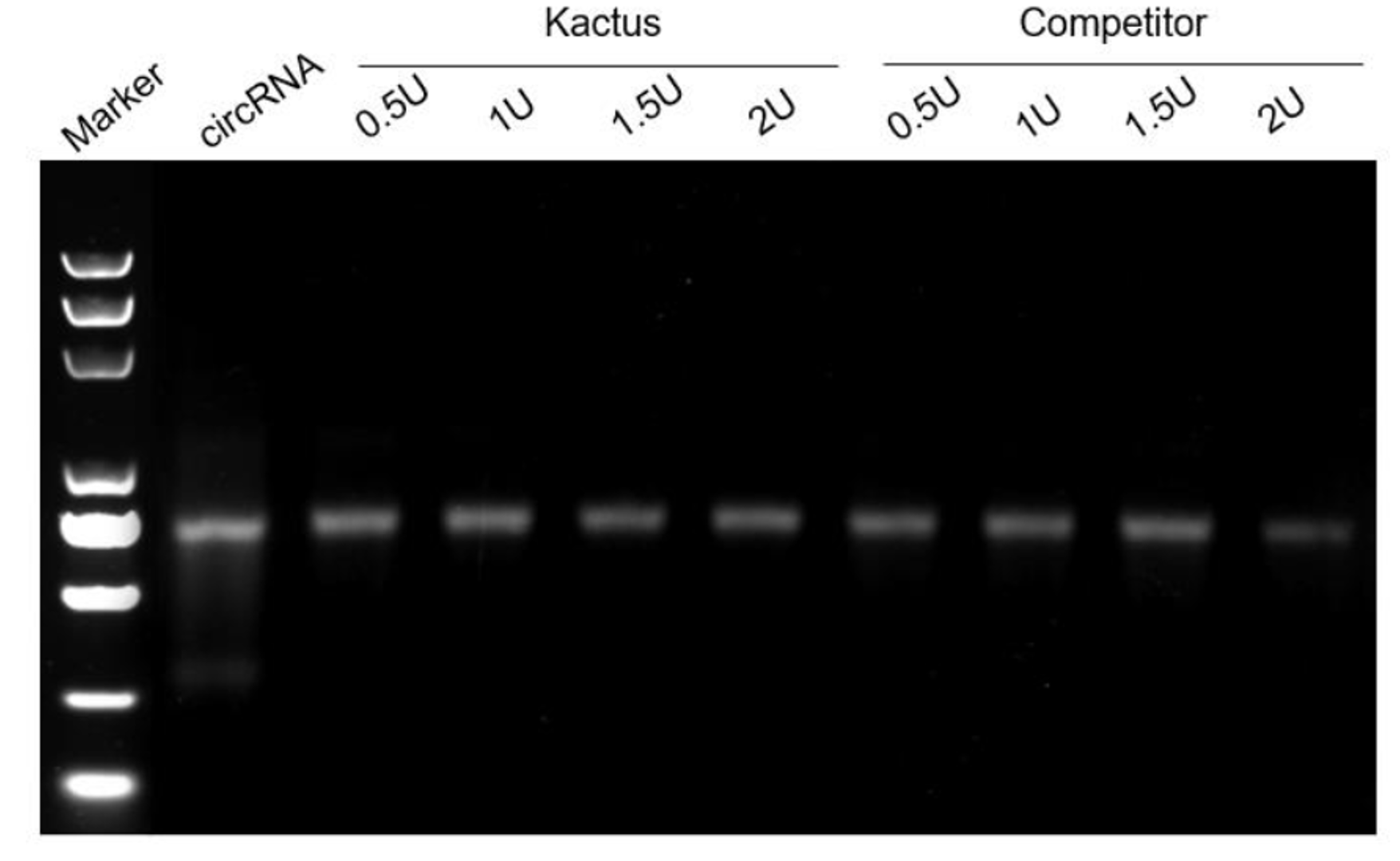
Figure 5. RNase R digests linear RNA (left) without affect circRNA (right).
Available Products
GMP-Grade: Currently manufactured according to cGMP guidelines.
GMP-Ready: Industrial-Grade enzyme that can be transitioned to our 100,000 sq ft GMP-Grade facility with GMP documentation and testing.
Drug Master Files: Submitted to the FDA Drug Master Files (DMF)

GMP-Grade Production

About KACTUS

Quality Control

SAMS™ Technology Platform
Enzymes for RNA Production FAQs
Explore frequently asked questions about enzymes used in mRNA production, covering enzyme functions, QC standards, GMP classification, and regulatory support for therapeutic manufacturing.
In vitro mRNA synthesis involves multiple enzyme types, including restriction enzymes for plasmid linearization, T7 RNA polymerase for transcription, capping enzymes, tailing enzymes, and ligases or exonucleases for circular RNA processing.
Linearizing plasmids ensures the production of full-length transcripts with defined 3' ends. Circular templates can lead to undefined or extended mRNA products, which can interfere with downstream processing.
T7 RNA Polymerase catalyzes the synthesis of RNA from a linearized DNA template. It is the central enzyme in IVT reactions used to produce synthetic mRNA and saRNA.
MaxPure™ T7 RNA Polymerase reduces dsRNA byproducts during transcription, improving mRNA purity. This is achieved through enhanced enzyme engineering for improved template processing.
The 5’ cap and 3’ poly(A) tail improve mRNA stability and translation efficiency while reducing innate immune responses. Capping enzymes and Poly(A) Polymerase introduce these modifications post-transcription.
Capping rate and poly(A) tail length are quantified using LC-MS analysis. Batch release includes assessment of these parameters to ensure consistency across enzyme lots.
Circularization can be achieved using self-splicing introns or RNA ligases such as T4 RNA Ligase I and II. RNase R is then used to digest linear RNA, enriching for circRNA.
GMP-Grade enzymes are produced under cGMP conditions with full QC and documentation. GMP-Ready enzymes are manufactured at industrial scale and can be transitioned to GMP production upon request.
Each enzyme batch is tested using substrate-specific assays, including plasmid digestion, RNA synthesis, or LC-MS-based structural analysis. Parameters such as unit definition and byproduct levels are recorded.
Yes. Selected RNA enzymes—including T7 RNA Polymerase, BsaI, and DNase I—have Drug Master Files (DMFs) registered with the U.S. FDA, supporting their use in regulated biomanufacturing environments.