Recombinant proteins designed & expressed by us.
As the sole manufacturer of our products, we have total control at every stage.
We design and manufacture our products in-house, giving a high level of control over every step from protein engineering to product validation. We test each product for bioactivity throughout the development process to ensure function in multiple in vitro settings. Each batch undergoes strict quality control testing including purity, activity, and endotoxin. Our high level of control results in superior batch to batch consistency. Moreover, the majority of our products are expressed using mammalian cell lines, allowing for post-translational modification, native protein conformation, and low endotoxin levels.
Quality Control
Quality control is a critical aspect of recombinant protein production that ensures the purity, potency, and consistency of the final product. Our quality control systems are designed to monitor and assess the quality of the production process and final product.
Quality Assurance
Our quality management system monitors the entire production process to ensure each batch is manufactured under the same optimal conditions for that protein. This includes monitoring the growth of the host cells, expression of the target gene, and purification of the recombinant protein. Moreover, the protein expression process is optimized during development to minimize the presence of contaminants that can affect the purity or activity of the final product.
Process Validation
We monitor the entire production process to ensure expression conditions are optimal for that specific protein. This includes monitoring the growth of the host cells, expression of the target gene, and purification of the recombinant protein. The expression process is optimized to minimize the presence of contaminants that can affect the purity or activity of the final product.
Analytical Testing & Equipment
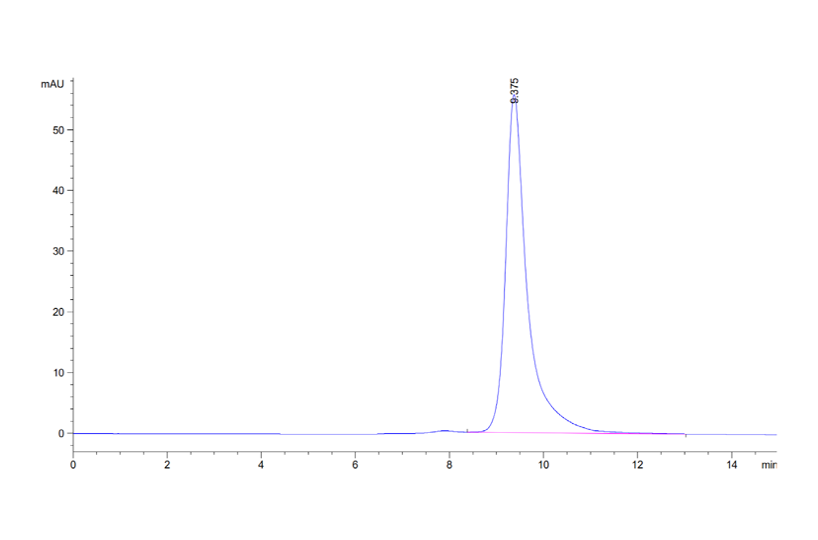
Purity
The purity of each recombinant protein is ensured using HPLC and by quantifying the level of impurities, such as host cell proteins, endotoxin, and host cell DNA in the final product.
Activity
The potency of our recombinant proteins is analyzed by measuring its in vitro activity, such as its ability to bind to its target molecule via ELISA or SPR assay.
Consistency
Our products are tested for batch-to-batch consistency to ensure stable bioactivity across lots. Additionally, we assess stability of our products by measuring degradation over time and resistance to environmental stress factors, such as temperature and pH changes.
CytoFlex SRT Flow Sorter

Biacore T200

6545XT Q-TOF Mass Spectrometer

Microplate Reader

Capillary Electrophoresis

1260 High Performance Liquid Chromatography
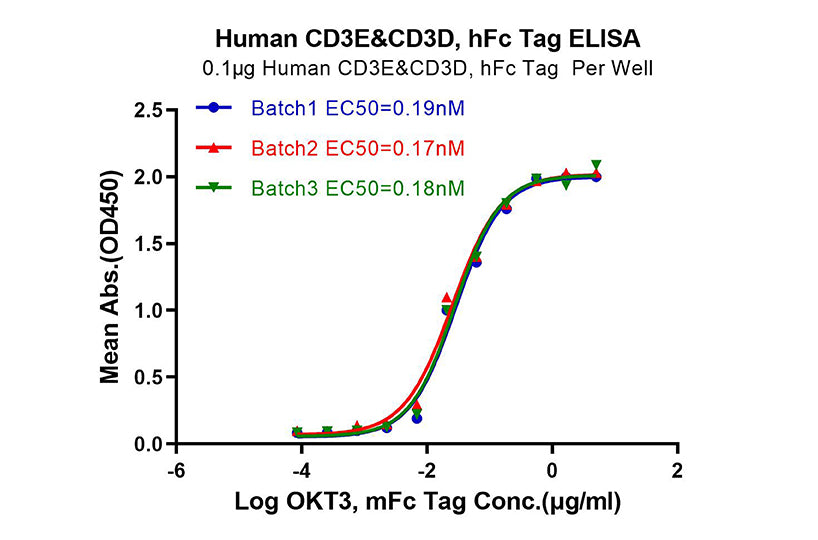
Reproducible results in your research with consistent activity across batches.
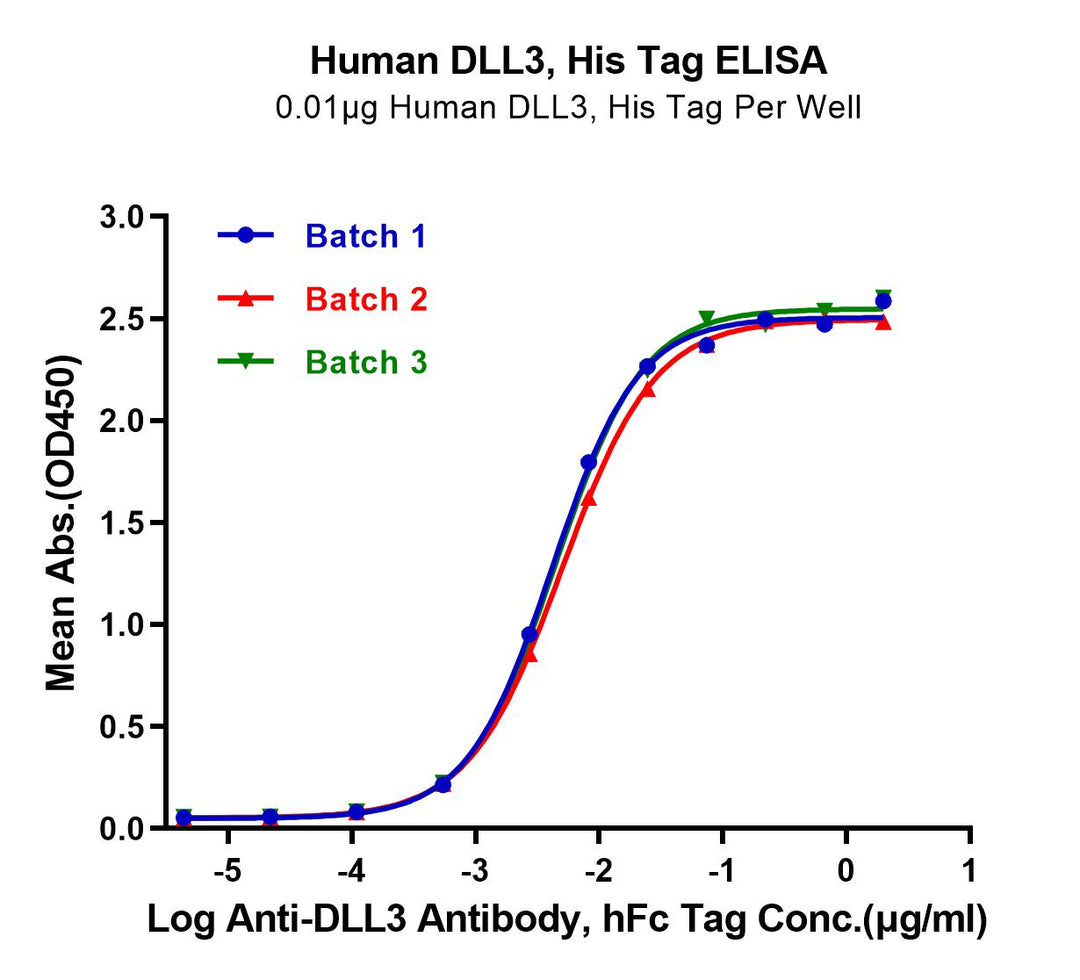
Immobilized Human DLL3, His Tag at 0.1μg/mL (100μL/well) on the plate. Dose response curve for Anti-DLL3 Antibody, hFc Tag with an EC50 of 5.0, 4.5, and 5.0ng/mL, respectively, as determined by ELISA.
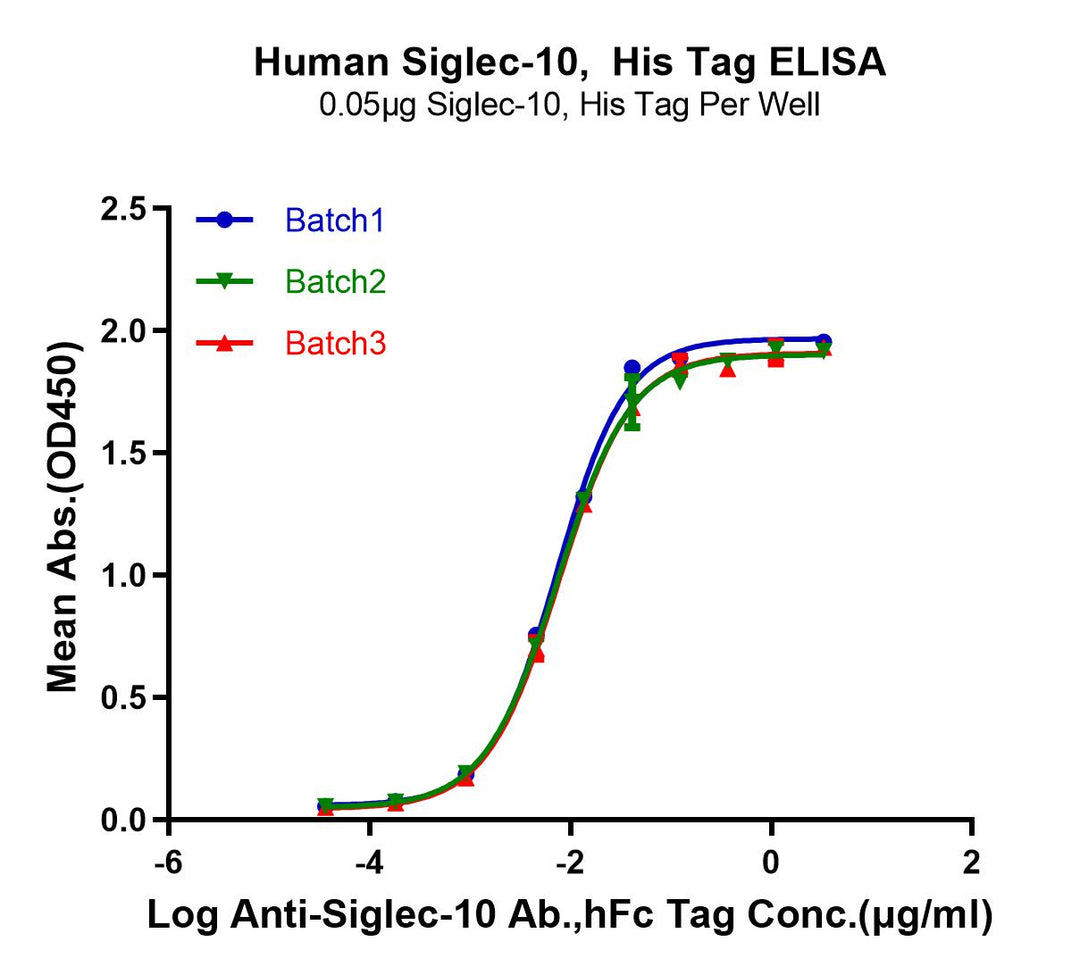
Immobilized Human Siglec-10, His Tag at 0.5μg/mL (100μL/well). Dose response curve for Anti-Siglec-10 Ab., hFc Tag with an EC50 of 7.3, 7.7, and 7.4ng/mL, respectively, asdetermined by ELISA.
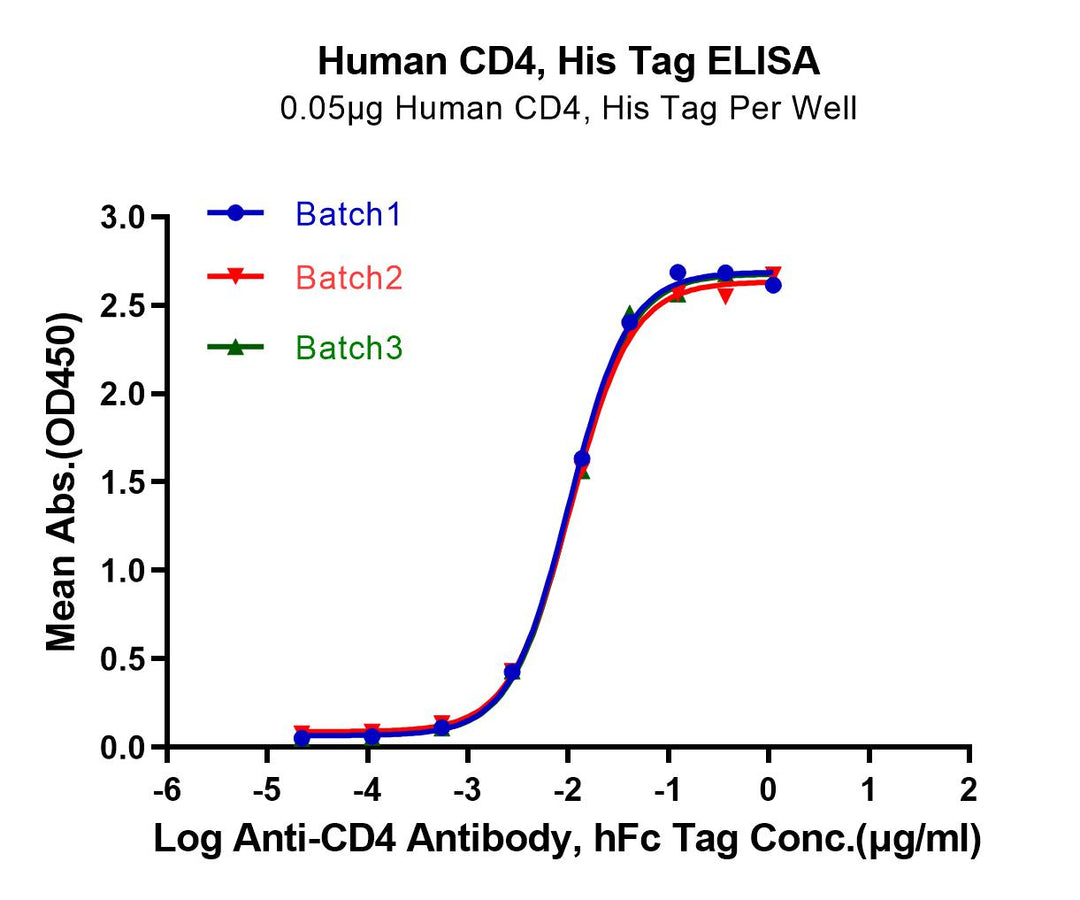
Immobilized Human CD4 at 0.5μg/mL (100μL/well) on the plate. Dose response curve for Anti-CD4 Antibody, hFc Tag with an EC50 of 10.2, 10.6, 10.6ng/mL, respectively, as determined by ELISA.

MHC Monomers & Tetramers

VLP-Displayed Antigens

Gene Editing Enzymes

Cytokines

CD3 Proteins
Frequently Asked Questions
Quality control (QC) ensures each recombinant protein meets defined criteria for purity, bioactivity, identity, and safety. It involves analytical testing to detect impurities, confirm structural integrity, and verify functional performance.
Purity is assessed using validated methods such as SEC-HPLC, SDS-PAGE, and capillary electrophoresis. Additional assays quantify residual host cell proteins, host DNA, endotoxins, and other process-related contaminants.
Functional assays such as ELISA, flow cytometry, and surface plasmon resonance (SPR) are used to confirm that the protein binds its intended target and retains expected biological function.
Batch consistency ensures reproducibility across experiments and supports regulatory compliance. QC teams assess each lot for yield, purity, bioactivity, and stability under controlled and stress conditions.
QC focuses on post-production testing of final products. QA oversees the entire manufacturing workflow, from raw material sourcing to documentation, ensuring that all steps are reproducible, traceable, and compliant with internal and regulatory standards.
KACTUS uses optimized expression systems such as mammalian or E. coli platforms tailored to the protein's complexity to promote correct folding and post-translational modifications, reducing misfolded species and endotoxin levels. Our VLPs and nanodiscs ensure that transmembrane proteins retain their native structure.
Key instruments include Biacore T200 (SPR), Q-TOF mass spectrometers, CytoFLEX SRT (flow cytometry), capillary electrophoresis systems, and microplate readers. These tools enable precise quantification of structure, binding, and stability.
Process validation monitors upstream (e.g., cell culture, transfection) and downstream (e.g., purification, filtration) steps to ensure consistency across production runs. Parameters such as growth conditions, yield, and elution profiles are tightly controlled.
Accelerated and real-time stability testing evaluates protein integrity over time under various storage temperatures and buffer conditions. Results guide expiry dating and formulation refinement.
Membrane proteins, cytokines, MHC complexes, and VLP-displayed targets demand enhanced QC due to their structural complexity, functional sensitivity, and intended use in translational or preclinical workflows.