Background
Immune checkpoints are a series of molecules expressed on immune cells that regulate the degree of immune activation, preventing excessive activation of the immune system. Dysfunction in the expression or function of immune checkpoints is a significant cause of many diseases. Tumor cells often keep immune checkpoints activated, preventing antigens from being presented to T cells, thereby impairing the normal immune function of T cells and enabling immune evasion. Common immune checkpoints include CTLA-4 (cytotoxic T lymphocyte-associated antigen-4) and PD-1 (programmed death receptor-1), which are receptors on the surface of T lymphocytes.
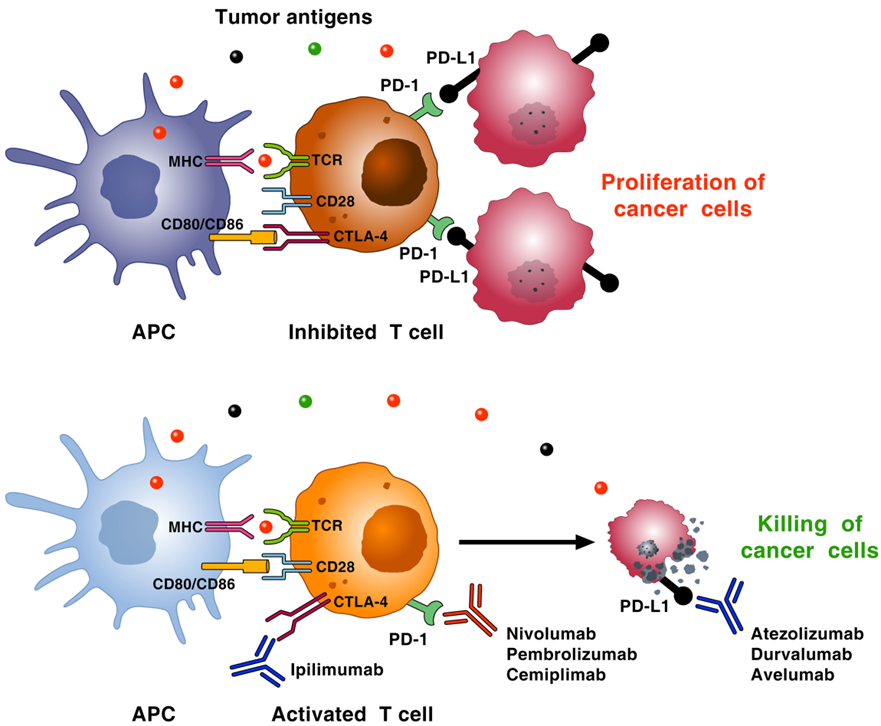
Mechanism of action of immune checkpoint inhibitors (Giusy Elia, et al, 2020)
Comprehensive Catalog of Immune Checkpoint Proteins
PD-1, PD-L1, CTLA-4, TIGIT, CD40 & more
Currently, immune checkpoints remain a hot topic in clinical research, with new targets and drugs emerging continuously. Based on our SAMS™ protein engineering and production platform, KACTUS has developed a series of immune checkpoint proteins with various tags, covering targets such as PD-1, PD-L1, CTLA-4, TIM-3, OX40, TIGIT, 4-1BB, and CD40. These proteins have undergone rigorous quality control and are suitable for antibody functional validation, cell function validation, pharmacokinetic analysis, and other disease-related research.
Product Features
→ HEK293 Expression
→ His, His-Avi, hFc Tags
→ Bioactivity verified (ELISA & SPR)
→ High Purity
→ Human, Mouse, Cynomolgus, Rhesus Macaque, Rat, Canine
→ Biotinylated Proteins
Product Applications
→ Antibody Discovery
→ Antibody Affinity Assays
→ Cell-Based Assays
→ Drug Discovery
→ Pharmacokinetics Analysis
Product Validation Data
Human TIGIT
TIGIT, a newly discovered immune checkpoint protein found on T cells and Natural Killer (NK) cells, plays a crucial role in modulating the immune response. As an inhibitory receptor, TIGIT acts as an "off switch" by competing with the activating receptor CD226 (DNAM-1) for ligand binding. This mechanism mirrors the relationship between CTLA4 and CD28, making TIGIT a promising target for cancer immunotherapy. TIGIT interacts with several ligands, including CD155 (PVR) and CD112 (PVRL2), with varying affinities. Through its inhibitory motif (ITIM), TIGIT suppresses T cell activation and NK cell cytotoxicity, contributing to immune homeostasis. Its ability to protect tumor cells from NK cell destruction makes it a target for enhancing anti-tumor immune responses.
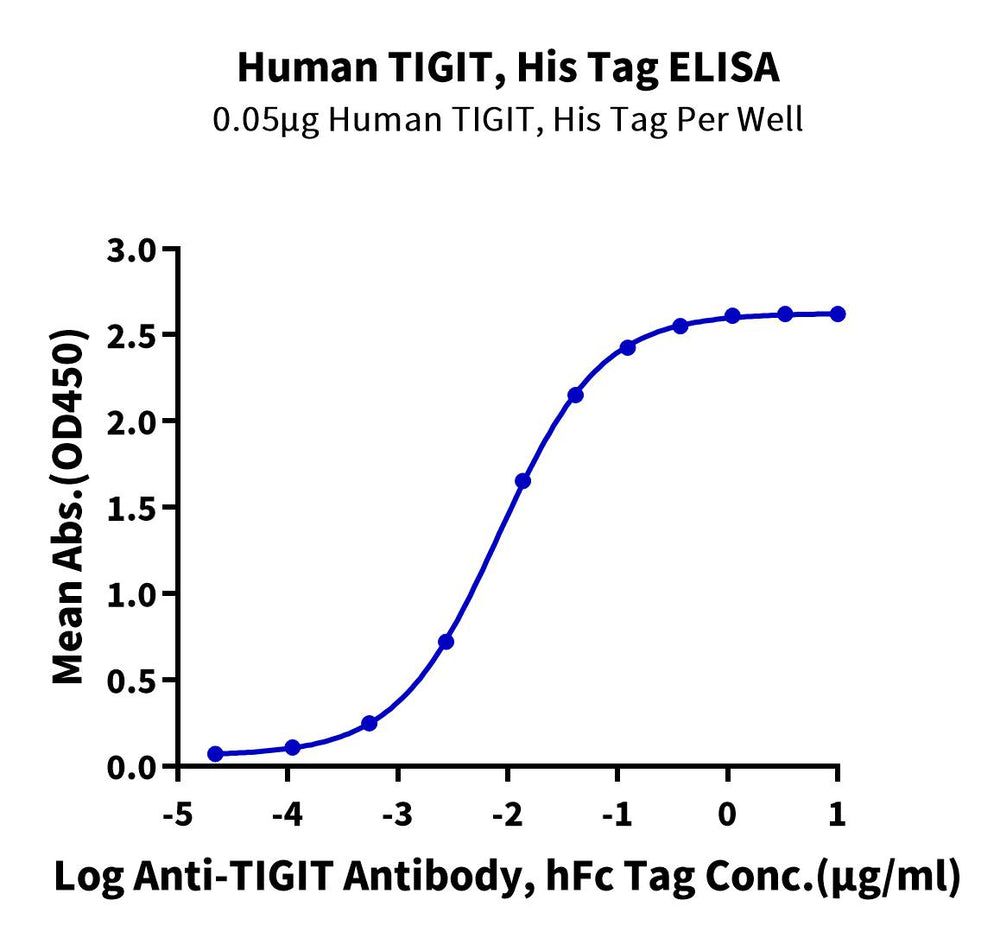
Human TIGIT, His tag was immobilized at a concentration of 0.5 µg/ml (100 µl/well) on the plate. A dose-response curve for the Anti-TIGIT Antibody with an hFc tag was generated, showing an EC50 of 8.3 ng/ml as determined by ELISA (QC Test).
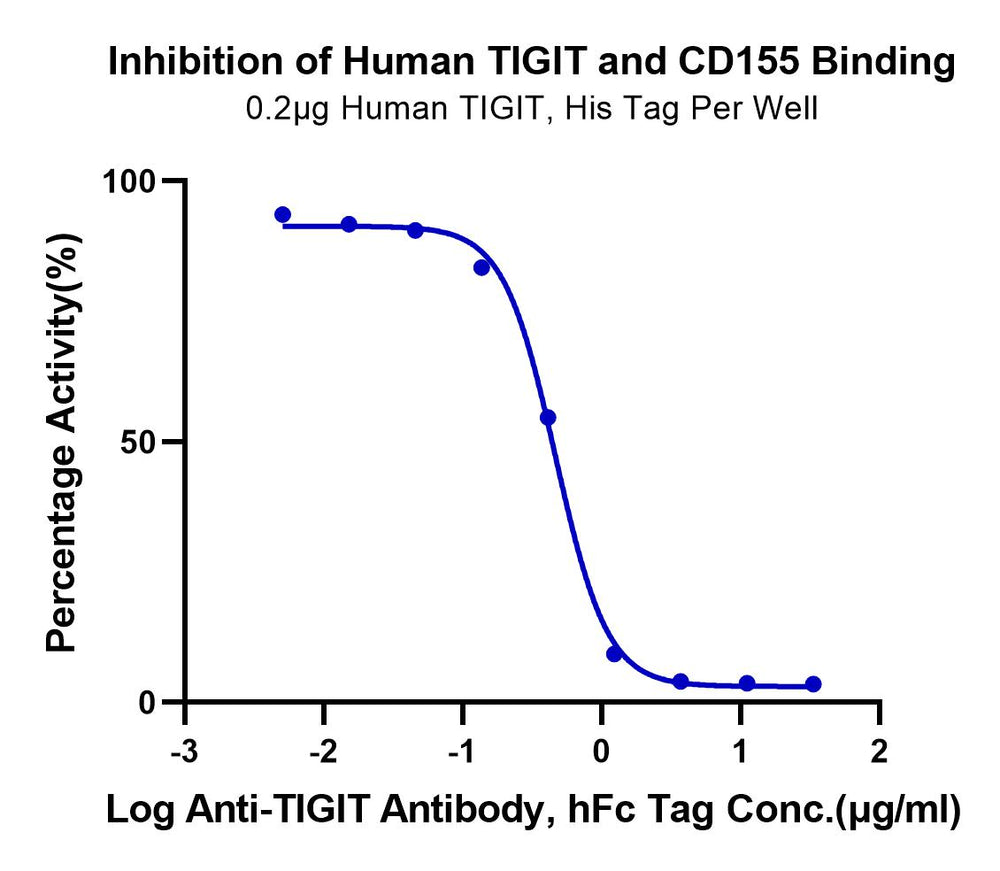
ELISA demonstrates that the binding of Human TIGIT to CD155 can be specifically blocked by the Anti-TIGIT Antibody. Serial dilutions of the Anti-TIGIT Antibody were added to the binding reactions of Human TIGIT with a His tag and Biotinylated CD155 with an hFc tag. The half-maximal inhibitory concentration (IC50) for this inhibition is 0.46 μg/mL.
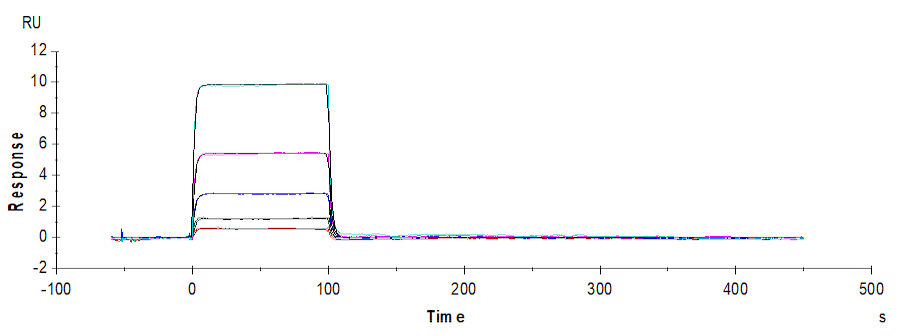
SPR analysis shows that Human TIGIT, His Tag, can specifically bind to Human CD155, hFc Tag captured on Protein A chip, with an affinity constant of 0.17 μM (Biacore T200).
Available Products

Customize your protein

About KACTUS

SAMS™ Technology Platform

Quality Control
Immune Checkpoint Proteins FAQs
Below are some of the most frequently asked questions about our immune checkpoint protein products, their applications, and customization options.
Immune checkpoint proteins regulate immune cell activity, maintaining immune balance and preventing autoimmunity. In cancer, tumors exploit these pathways to evade immune responses, making them critical targets in immunotherapy research.
KACTUS offers many recombinant immune checkpoint targets including PD-1, PD-L1, CTLA-4, TIM-3, TIGIT, OX40, 4-1BB, and CD40. Proteins are available across species: human, mouse, cynomolgus macaque, rhesus macaque, rat, and canine.
All immune checkpoint proteins are validated using ELISA and SPR assays to confirm binding affinity and receptor-ligand functionality. IC50 or ED50 values are included when applicable.
KACTUS checkpoint proteins support a range of applications, including:
– Antibody discovery
– Functional cell-based assays
– Drug screening and lead validation
– Pharmacokinetic (PK) and immunogenicity studies.
Tagged checkpoint proteins are available with His, His-Avi, monomeric hFc, or biotin labels. These formats are compatible with ELISA, SPR, flow cytometry, and other in vitro assays.
Yes. KACTUS offers species-specific proteins to support cross-reactivity studies and preclinical model testing in mouse, cynomolgus, and other non-human systems.
Yes. KACTUS supports customization requests such as tag changes, biotinylation, or fluorescent labeling. Contact our support team to request tailored modifications.
These products are for research use only (RUO). However, they are produced using high-quality expression systems and QC-tested, making them suitable for preclinical assay development, PK testing, and early drug research workflows.
Refer to the product-specific datasheet for guidance. Most checkpoint proteins are shipped lyophilized or frozen, and should be reconstituted and aliquoted to prevent repeated freeze-thaw cycles.
KACTUS checkpoint proteins combine proprietary SAMS™ protein engineering, HEK293 expression, and bioactivity validation to deliver high-purity proteins with reliable functional performance for immuno-oncology research.