Empower Your Research with Precision DNA Enzymes
At KACTUS, we are dedicated to pushing the boundaries of gene editing technology. Our commitment to innovation and excellence drives us to develop and provide the highest quality gene editing enzymes, including Cas9, Cas12a, and AccuBase®. These cutting-edge tools empower researchers and scientists to achieve precise and efficient gene modifications, paving the way for groundbreaking advancements in genomics, therapeutics, and beyond. Join us on our mission to transform the future of gene editing and unlock new possibilities in the life sciences.
Download Product Brochure:
AccuBase®
The Next Generation Base Editor
AccuBase® is a cytosine base editor engineered by Base Therapeutics and manufactured for global sales by KACTUS. It creatively embeds a deaminase inside the Cas protein to prevent random binding of deaminase to non-target sites, significantly reducing off-target occurrence while still maintaining high editing efficiency. Using our protein engineering and expression platform, Structure Aided Multiplex Screening (SAMS™), KACTUS has successfully manufactured the DNA base editor. We've maximized the stability, purity, and activity of AccuBase® by screening our protein expression system, optimizing the purification process, and performing formulation development. AccuBase® is a cytosine base editor which can convert a C•G base pair into a T•A base pair.
Learn More → View the brochure
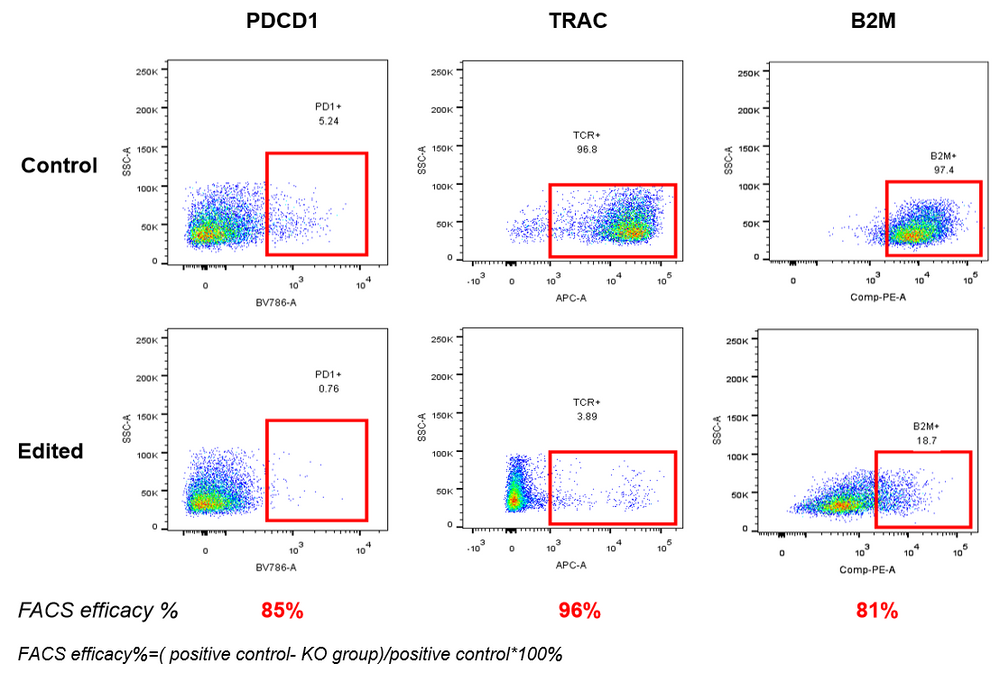
AccuBase® RNP was electroporated into activated primary T cells. According to flow cytometry, AccuBase® can efficiently knock out PD, B2M, and TRAC proteins on the membrane of activated primary T cells at the protein level. For PD1 and B2M, the knockout efficiency exceeded 80%, while the knockout efficiency of TRAC reached 96%.
CRISPR Cas9 Protein
The classic CRISPR Workhorse
KACTUS has successfully developed a highly active GMP-grade Cas9 nuclease based on our Structure Aided Multiplex Screening (SAMS™) technology platform. Our Cas9 Nuclease is the wild-type sequence that has undergone codon optimization, nuclear localization signal (NLS) design, and optimization of E. coli expression and purication. Our GMP-Grade Cas9 Nuclease is available in long-term bulk supply with consistent batch activity, to ensure a smooth transition while scaling up your drug production from development to manufacturing.
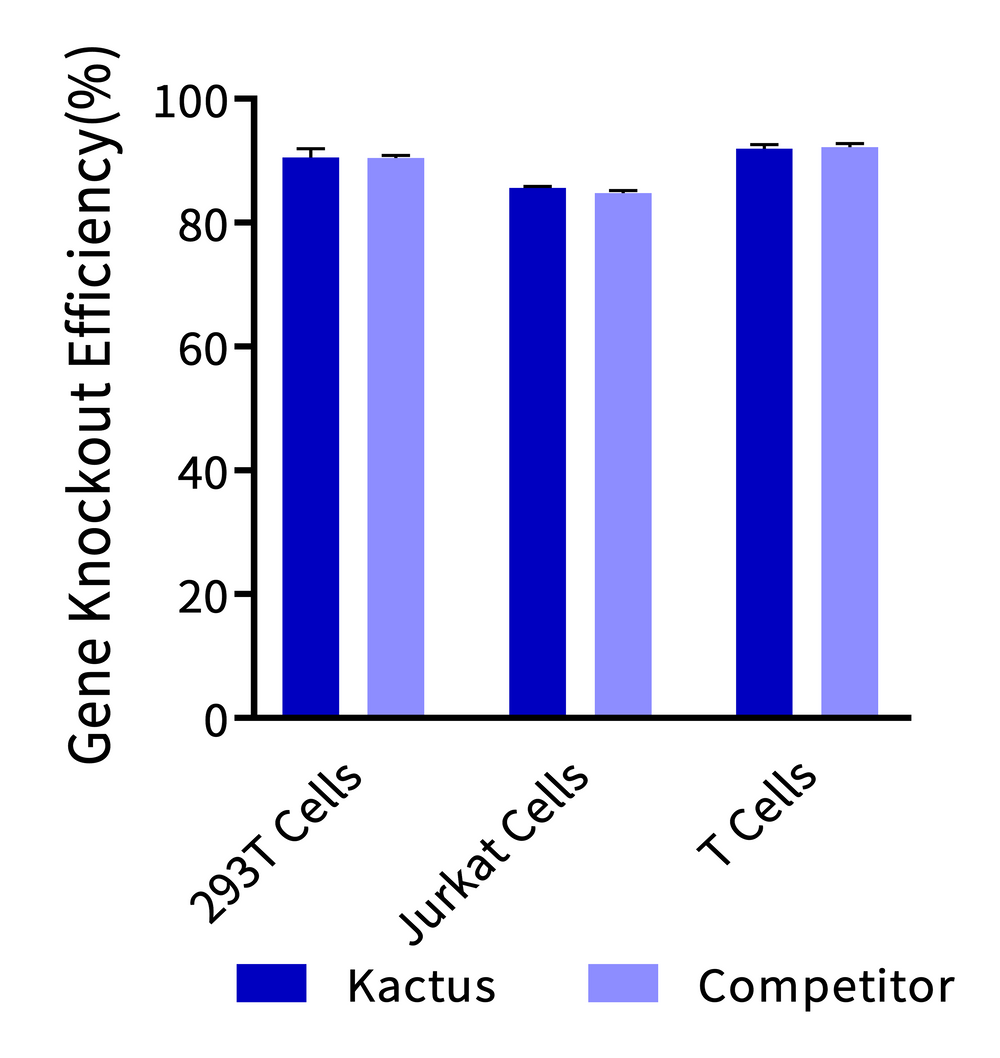
Gene knockout efficiency was analyzed using three batches of KACTUS GMP-Grade Cas9. Knockout of BCL11A, BCL11A, and TRAC gene was analyzed for 293T, Jurkat, and T cells, respectively. The cells were electroporated with 75 pmol of Cas9 protein along with 225 pmol of sgRNA into three cell types. TIDE analysis was performed after three days of culture post-electroporation. Results show greater than 85% editing efficacy across all three cell types, comparable to industry leading supplier.
Cas12a
The Versatile Gene Editor
KACTUS provides a highly active AsCas12a nuclease. Different from Cas9 nuclease, AsCas12a targets T-rich sequences and forms 4-5 nucleotide protruding 5' sticky ends after cutting double-stranded DNA.
Our Cas12a enzyme is manufactured research-grade but can be moved to GMP-Grade production standards upon agreement.
Learn more about our GMP enzyme production or contact us to discuss your specific requirements.

Verified by in vitro cleavage experiments, AsCas12a nuclease can cleave more than 95% of DNA substrates.

GMP-Grade Production

About KACTUS

Quality Control

SAMS™ Technology Platform
Gene Editing Enzymes FAQs
KACTUS provides Cas9, Cas12a (AsCas12a), and AccuBase® cytosine base editor enzymes. Each enzyme supports different genome editing strategies based on application needs and sequence context.
– Cas9 recognizes NGG PAM sites and generates blunt-ended double-strand breaks
– Cas12a targets T-rich PAMs and creates 5′ staggered cuts, leading to different DNA repair outcomes
Cas12a also uses a single crRNA, offering simplified guide design compared to Cas9.
AccuBase® is a cytosine base editor that converts C•G to T•A without inducing double-strand breaks. Its embedded deaminase remains inactive until the correct DNA target is bound, helping reduce off-target activity compared to standard nucleases like Cas9.
Delivered as an RNP complex into activated human T cells, AccuBase® shows:
– >80% knockout for PD1 and B2M
– 96% knockout for TRAC
These results are based on flow cytometry analysis in primary cell assays. Efficiency varies depending on the target gene.
Cas9 and AccuBase® are available in both research-grade and GMP-grade. Cas12a is currently available as research-grade, with GMP-grade available upon request.
Cas9 and AccuBase® are expressed in E. coli and undergo optimized purification. Cas9 includes a nuclear localization signal for efficient transport into eukaryotic cells.
Each batch is tested for in vitro cleavage activity, purity by SDS-PAGE and SEC-HPLC, and consistency across production lots. Knockout efficiency is verified in 293T, Jurkat, and primary T cells.
Batches are assessed for editing efficacy, enzyme purity, and storage stability. GMP documentation includes batch records, CoAs, and validation reports in support of regulatory submissions.
Yes. GMP-grade Cas9 and AccuBase® are designed for use in regulated environments, including IND-enabling studies and manufacturing of gene and cell therapy products.
SAMS™ (Structure Aided Multiplex Screening) is KACTUS' proprietary protein engineering platform. It supports the optimization of expression, folding, and functional performance of recombinant proteins, including gene editing enzymes, ensuring high activity and scalability.





