Overview
Since the approval of the first antibody drug OKT3 in 1986, antibody drugs have occupied an important position in the pharmaceutical market. Currently, antibody drugs mainly come in various types such as monoclonal antibodies, bispecific antibodies, and XDCs (such as antibody-drug conjugates, ADCs). With the continuous development and improvement of monoclonal antibody technology, antibody drugs have become highly diversified in terms of target types, drug forms, and therapeutic areas. KACTUS, with its proprietary protein research and development production platform SAMS™, has launched various types of high-quality recombinant proteins to fully meet customer needs for antibody development.
Early Stage Discovery
In the early stages of antibody drug development, it is often necessary to use antigen proteins for animal immunization to obtain antibodies. KACTUS offers a wide variety of target proteins, all of which have undergone strict biological activity tests, aiding customers in animal immunization.
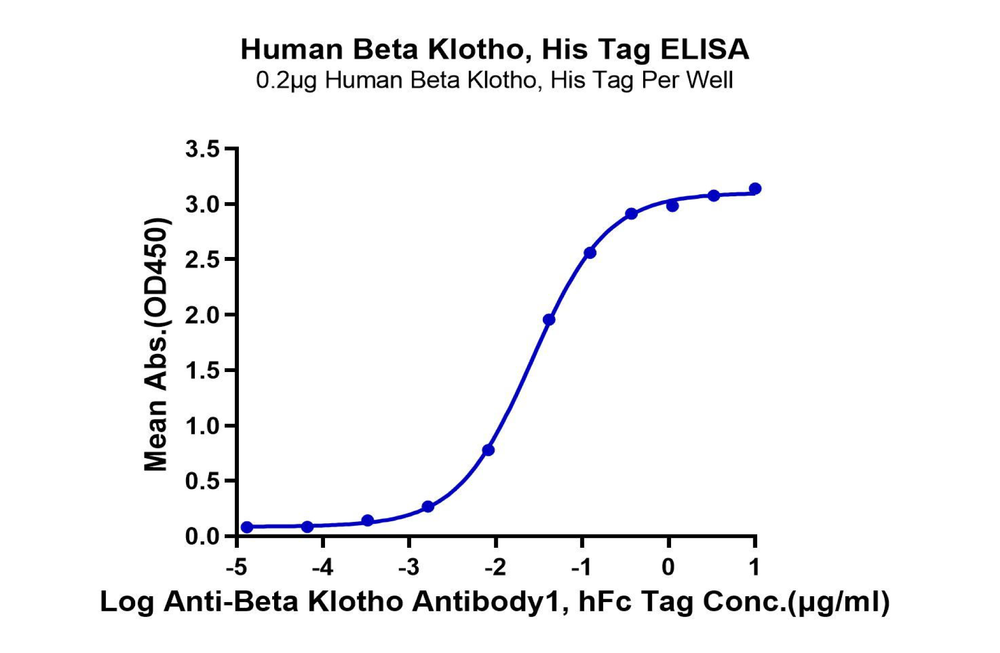
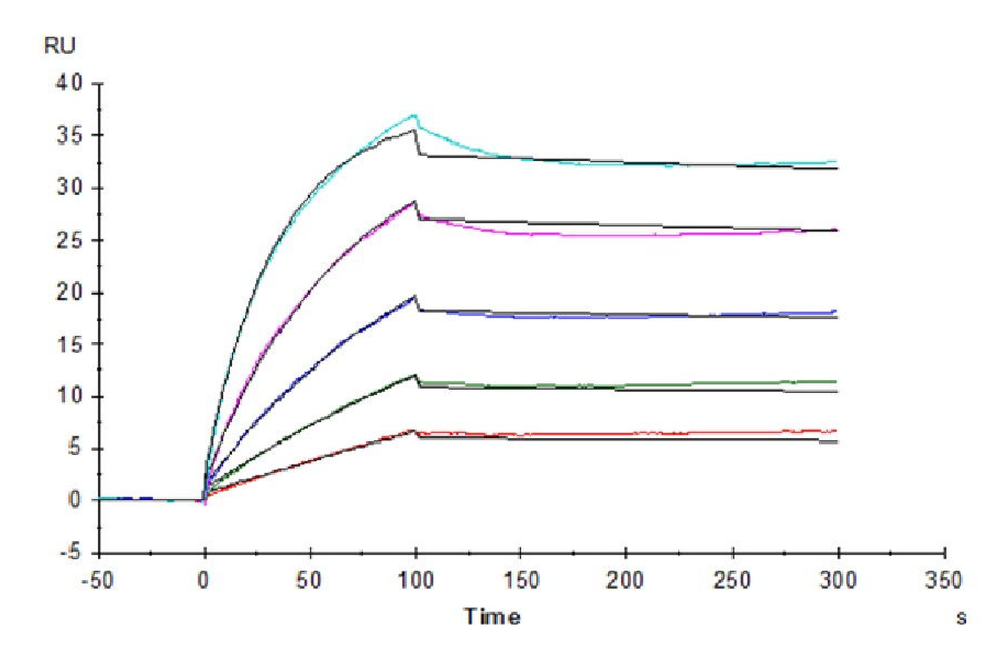
Figure 1. Our recombinant human β-Klotho protein exhibits excellent binding ability with its antibody (left graph, ELISA) and ligand FGF21 (right graph, SPR).
For multiple transmembrane protein targets, such as the GPCR family and the Claudin family, the presence of several hydrophobic regions makes it difficult to be solubly expressed and maintain activity. KACTUS adopts special designs, such as using virus-like particle (VLP) proteins for display, allowing soluble expression while maintaining the correct conformation and biological activity of the protein. Additionally, for proteins with poor direct immunization effects or weak immunogenicity, utilizing VLP display not only retains good biological activity but also achieves stronger immunization effects.
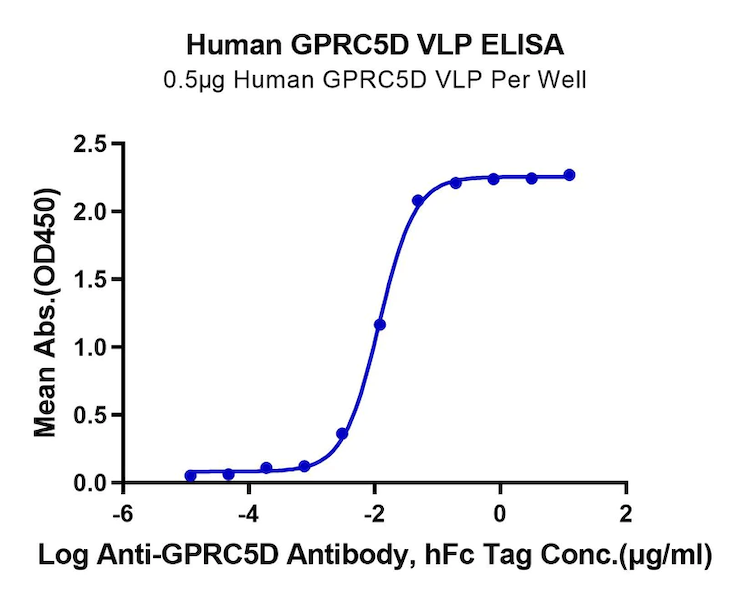
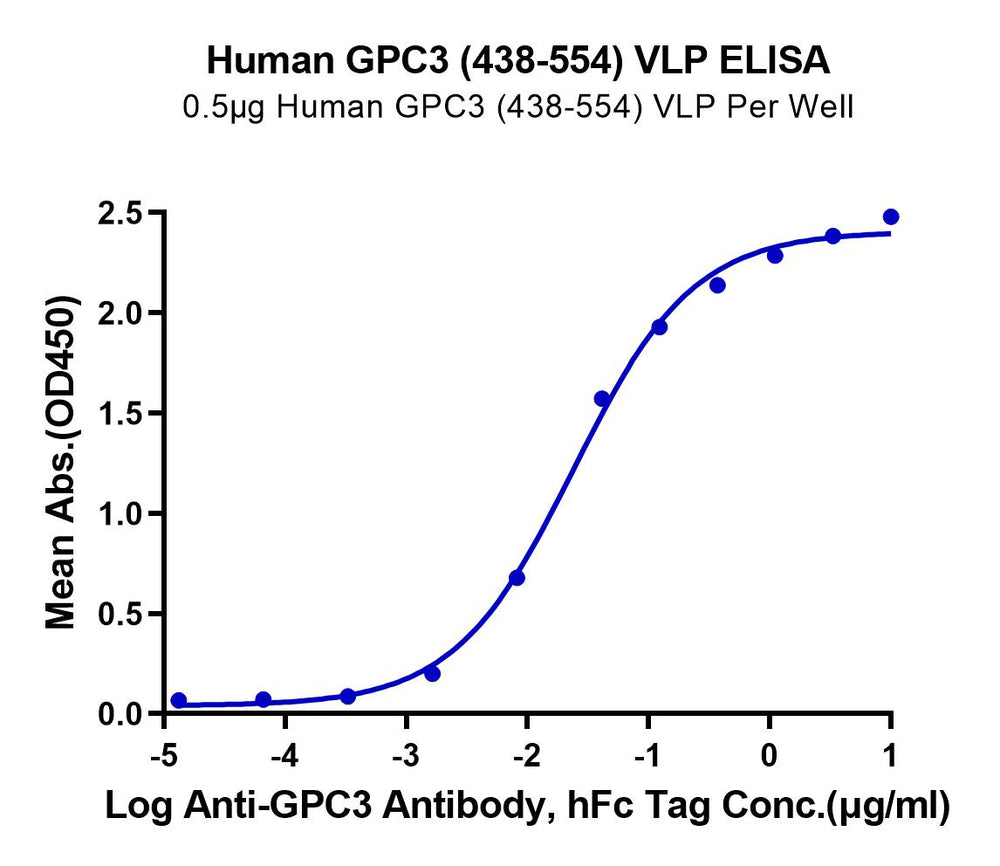
Figure 2. GPRC5D and GPC3, displayed using VLP, both exhibit good binding activity with their respective antibodies.
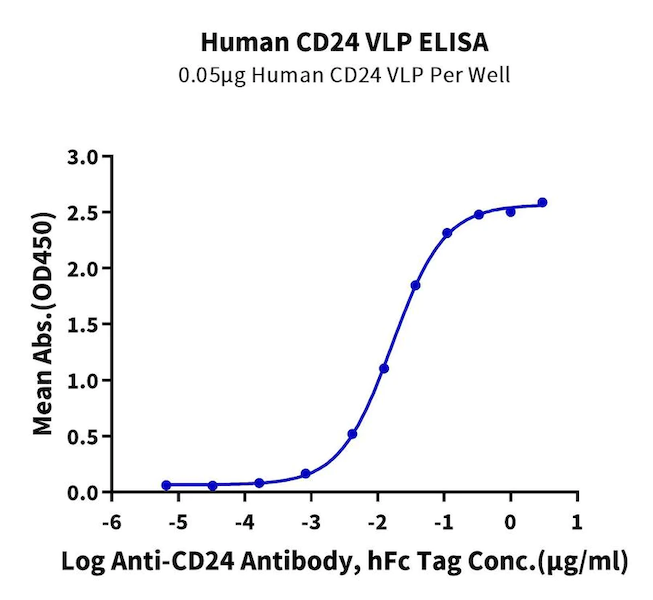
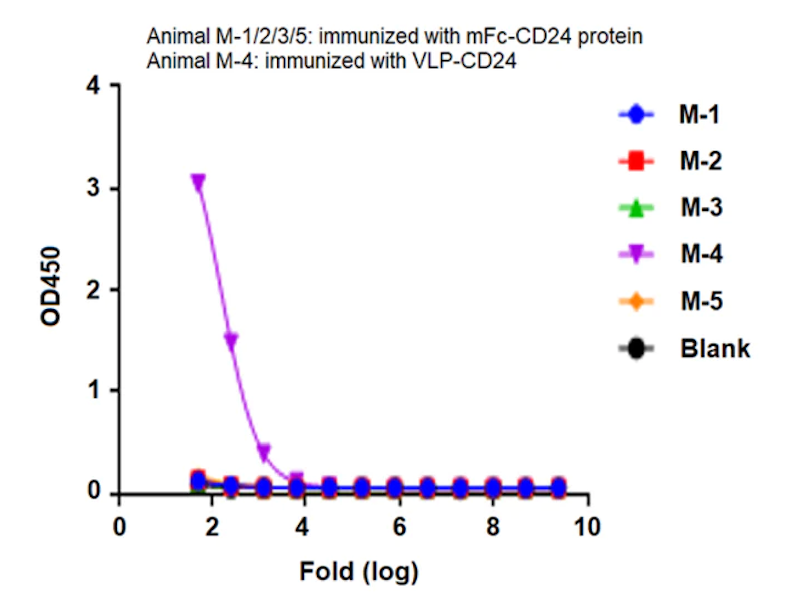
Figure 3. CD24, displayed using VLP, not only binds well with the antibody (left), but also shows a significant enhancement in immunization effects (right).
Featured Discovery Targets
Biotinylated & Fc Receptor Proteins for Antibody Screening
After obtaining antibodies, it is necessary to screen and optimize the acquired antibodies. Proteins labeled with biotin have higher sensitivity and specificity, which can be used not only for various detection analyses but also for immune capture. They have unique advantages in analyses like ELISA, SPR, flow cytometry, and biopanning.
KACTUS offers both site-specific and non-site-specific biotinylated proteins for customers to conduct antibody screening tests. Site-specific biotinylated proteins utilize the principle that the lysine residue on the Avi tag can be modified with biotin by the biotin ligase BirA, enabling precise control of biotin modification. Moreover, when the protein is fixed to the surface of the affinity-coated bead, it has directional consistency and is widely used in various detection analyses.
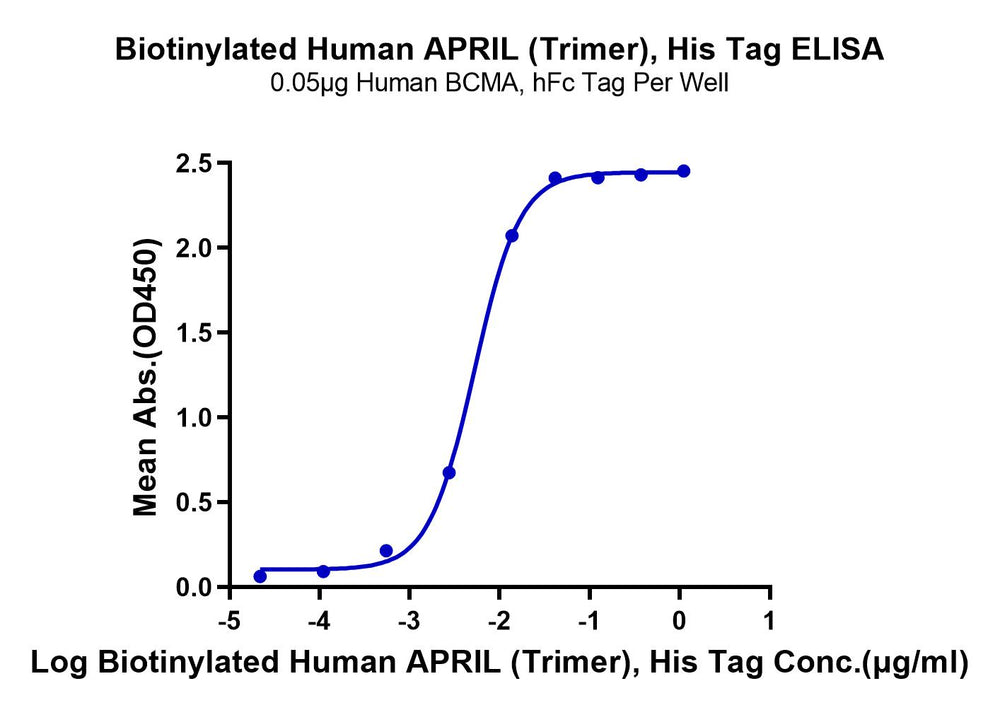
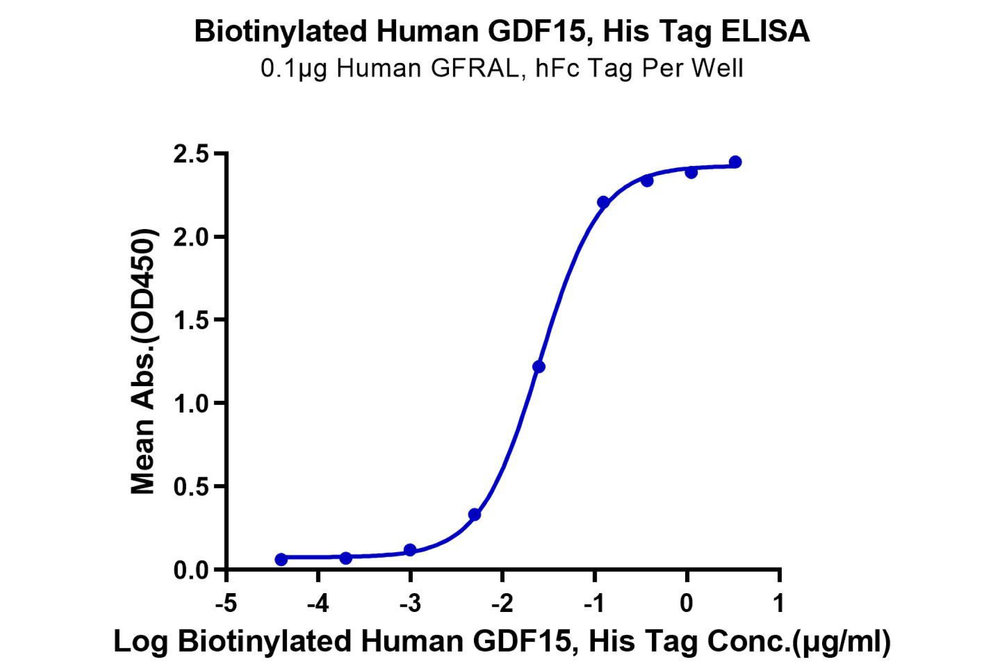
Figure 4. Verified by ELISA, site-specific biotin-modified Human APRIL (Trimer) (left) and GDF15 (right) both exhibit good binding activity with their respective ligands.
Non-site-specific biotinylated recombinant proteins are produced by chemical labeling, where the free amino groups or lysine residues of the protein can bind with biotin. This method may have higher sensitivity as multiple biotins can be labeled on each protein molecule.
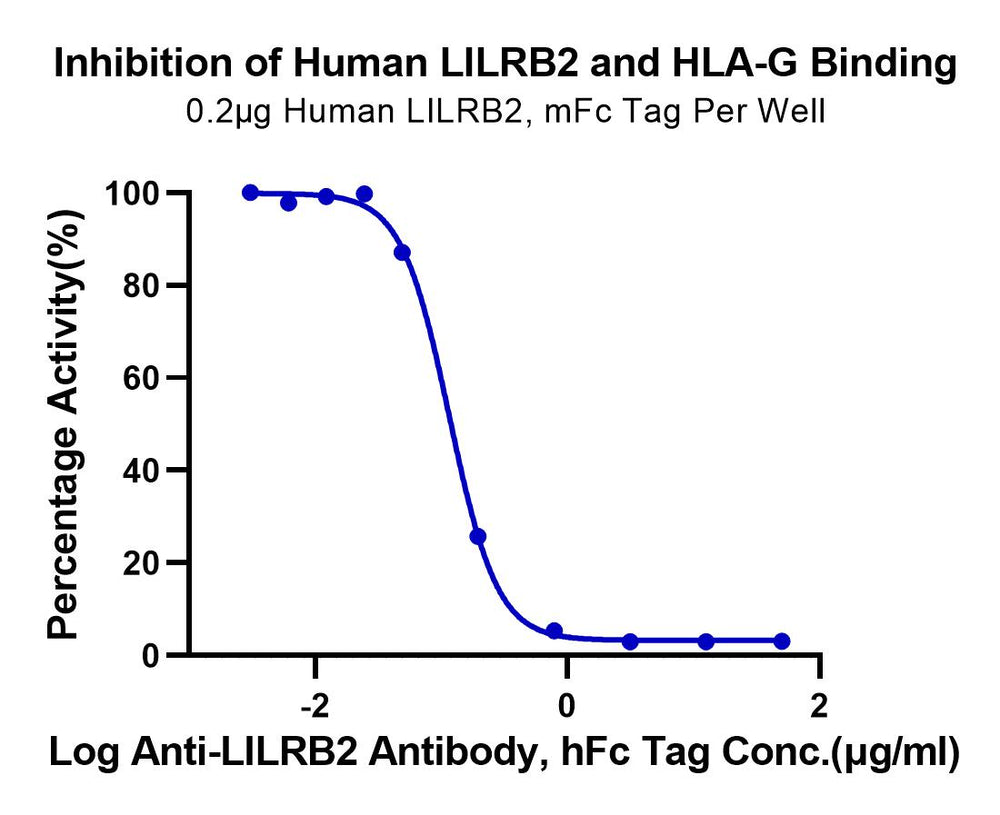
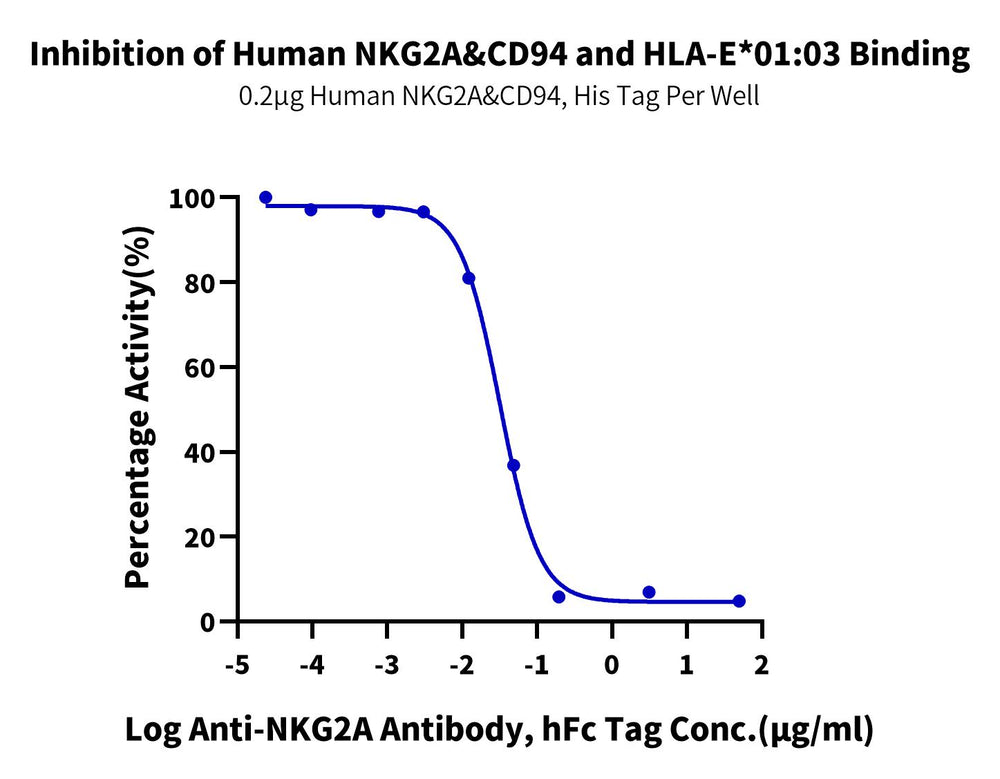
Figure 5. Verified by ELISA, non-site-specific biotinylated labeled HLA-G or HLA-E tetramers can be applied in blocking analysis experiments related to corresponding antibodies.
In addition, during the performance evaluation stage of antibody drugs, besides analyzing the binding situation between the antibody Fab fragment and the target, it is also necessary to analyze the binding ability of the Fc segment with the Fc receptor, to evaluate the antibody's half-life or the antibody’s ADCC effect. The Fc receptor protein series from KACTUS, including FcRn and various mutated forms of Fcγ receptors, can aid in antibody optimization and in obtaining antibodies with the desired affinity.
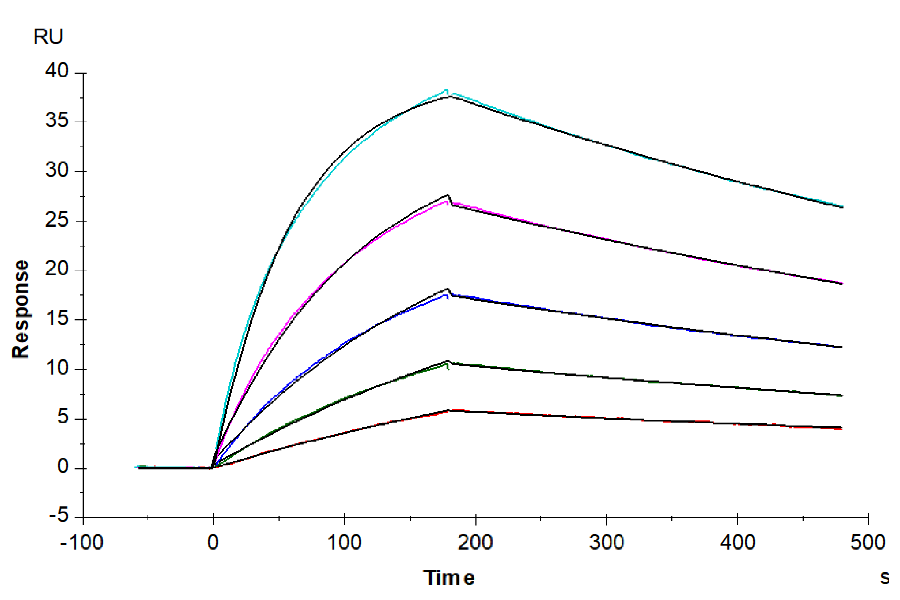
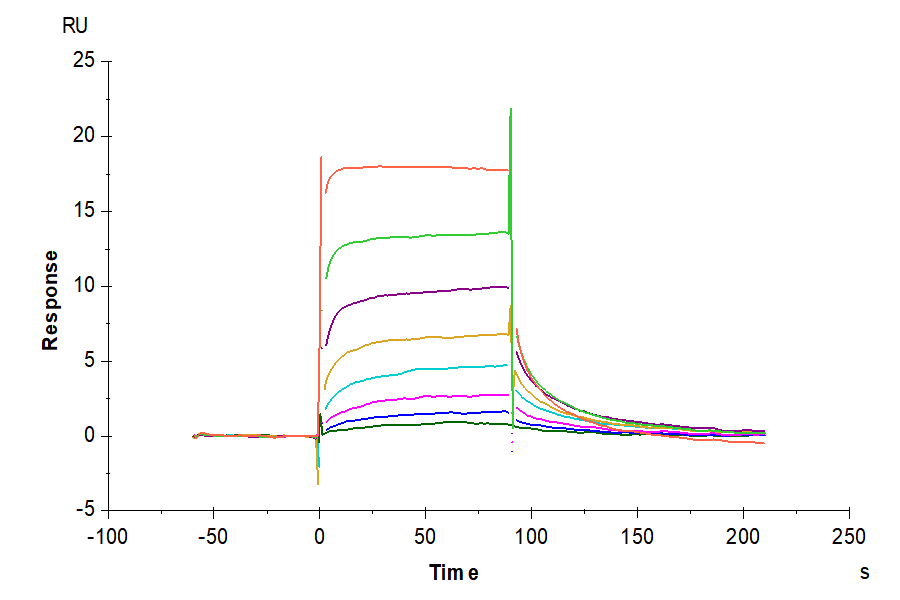
Figure 6. (Left) Verified by SPR, Trastuzumab can bind with Human Fc gamma RI, with an affinity constant of 1.94 nM. (Right) Verified by SPR, Human IgG1 can bind with Human FcRn, with an affinity constant of 0.28 µM.
Featured Biotinylated Proteins
Featured Fc Receptor Proteins
Target Proteins for CMC Method Development
CMC (Chemistry, Manufacturing, and Controls) is one of the key links for the successful development and registration of a drug, requiring high-quality proteins with high stability to establish production control parameters and imposing higher requirements on the stability and activity of the proteins. KACTUS' recombinant protein products possess high stability and excellent activity, which can be applied in the CMC method development of antibody drugs. Example data:
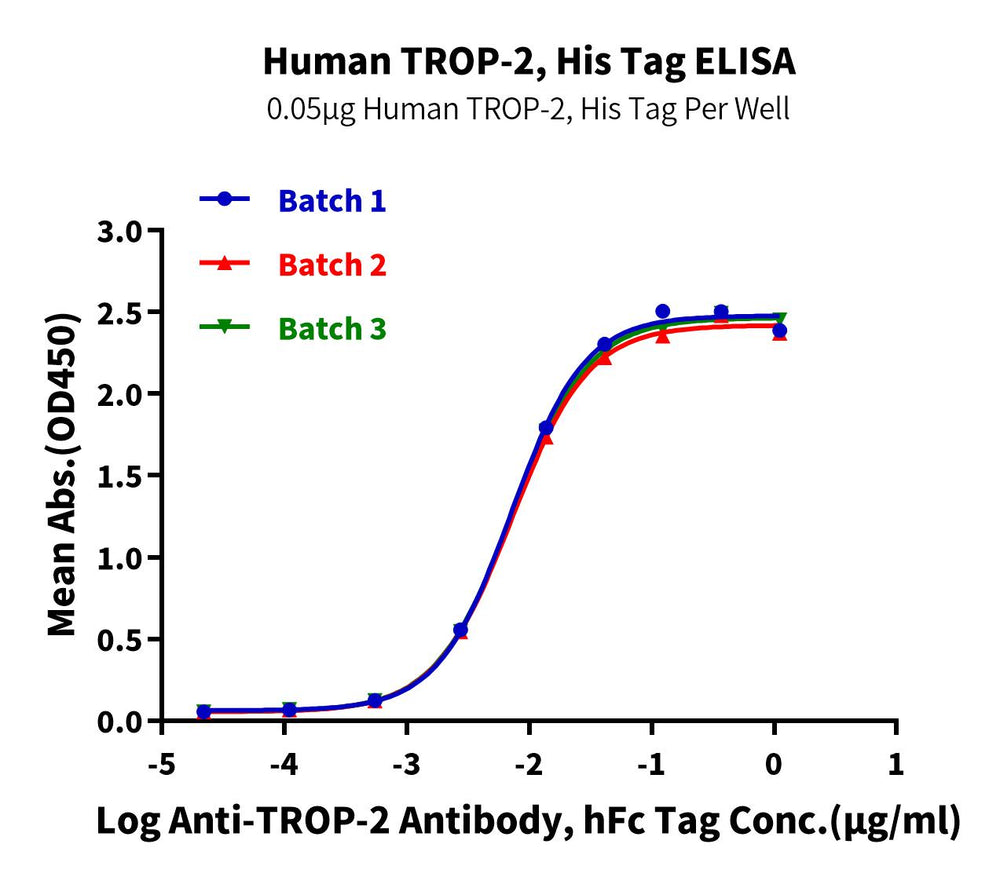
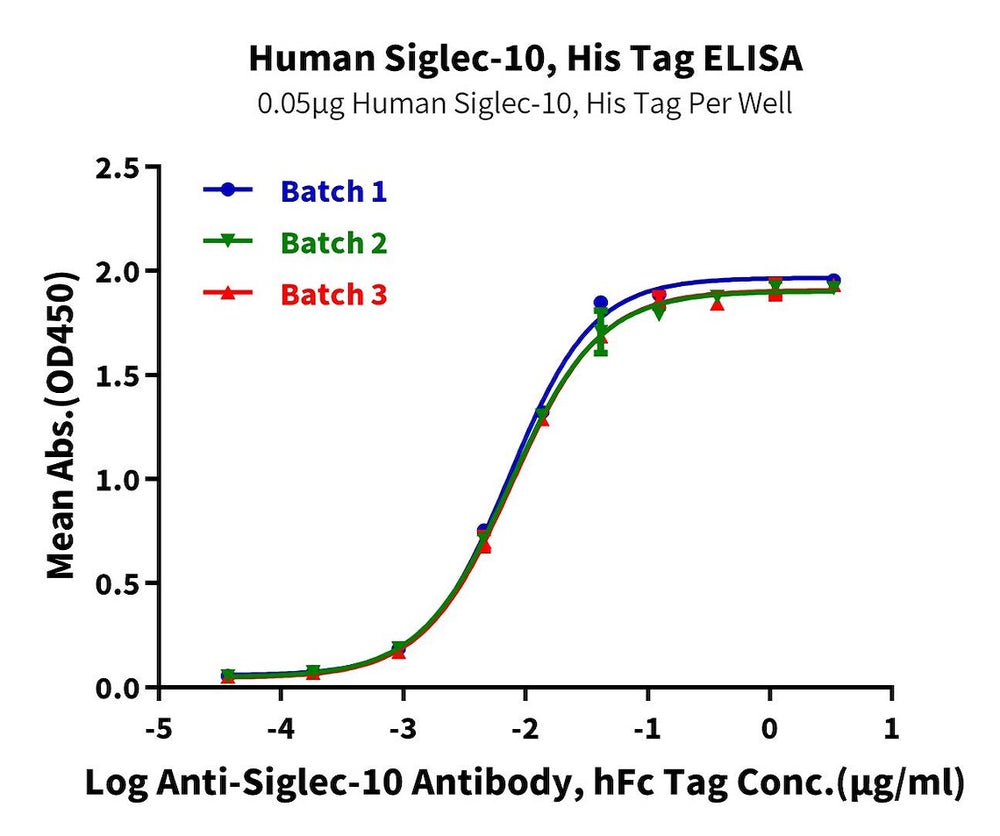
Figure 7. Verified by ELISA, recombinant Human Trop-2 (left) and Siglec-10 (right) proteins exhibit good batch-to-batch stability.
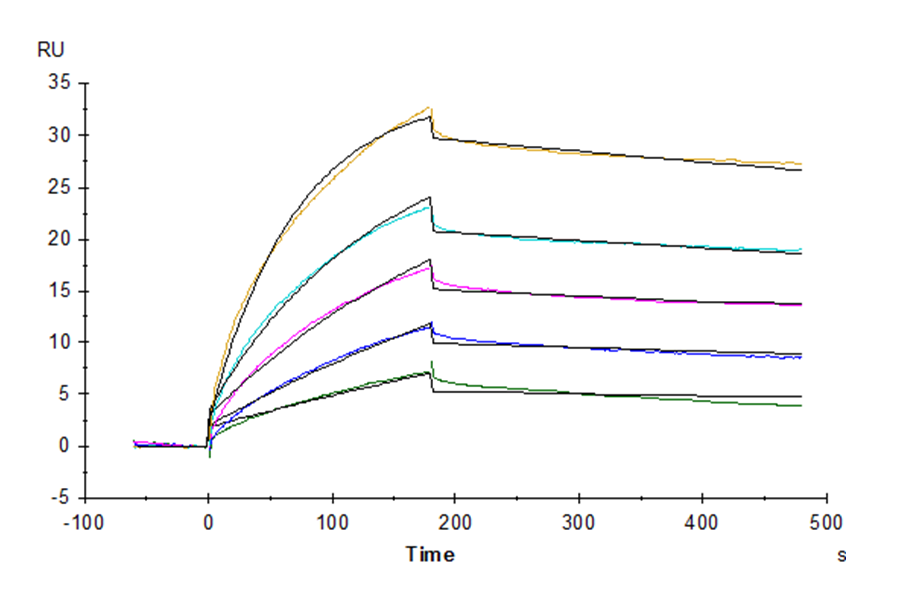
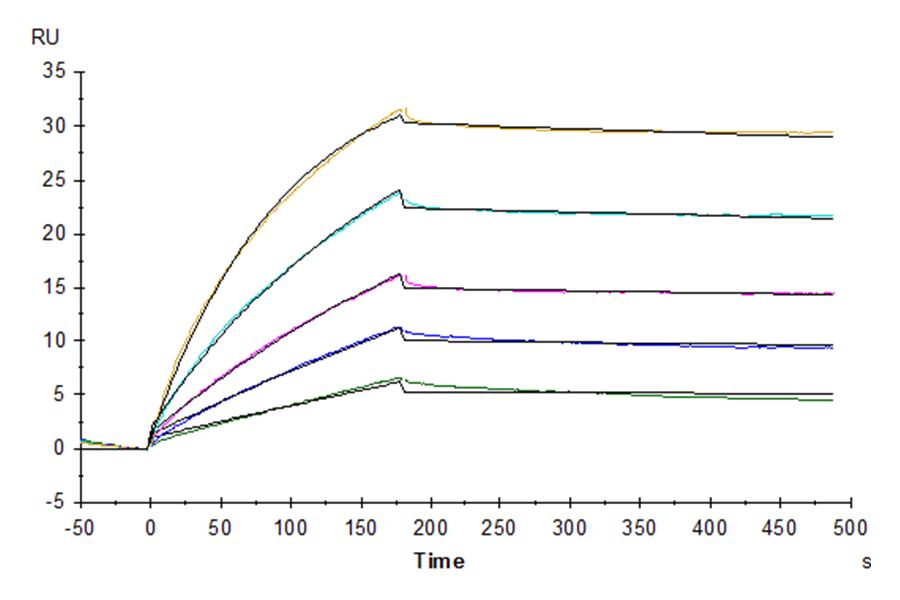
Figure 8. Verified by SPR, both biotinylated Claudin 6-VLP (left) and biotinylated CD20-VLP (right) proteins can bind well with their respective antibodies.
Featured Proteins for CMC Development

Customize your protein



Proteins for Antibody Drug Discovery FAQs
Find answers to common questions about our recombinant proteins used in antibody drug discovery, including applications in screening, immunization, and assay development.
Recombinant target proteins serve as antigens in the early stages of antibody drug discovery. They are used to trigger immune responses or act as binding targets for screening, selection, and validation of antibody candidates.
Protein quality is verified using functional assays such as ELISA and surface plasmon resonance (SPR). These tests confirm binding activity, structural integrity, and batch consistency.
Transmembrane proteins contain multiple hydrophobic domains, making them unstable in solution and challenging to express in functional form. Special strategies like VLP display are used to maintain their conformation.
VLP display supports proper protein folding and enhances immunogenicity, which can be especially useful for targets with weak immune responses or poor expression profiles.
– Site-specific biotinylation attaches biotin at a defined location, preserving protein orientation and activity.
– Non-site-specific biotinylation adds multiple biotins across the molecule, increasing signal intensity but sometimes affecting binding behavior.
Biotinylated proteins are used in ELISA, SPR, flow cytometry, and bead-based screening. They allow for targeted immobilization and high-sensitivity detection in binding and competition assays.
Fc receptor proteins are used to study interactions between the Fc region of antibodies and immune effector receptors. These interactions are critical for evaluating ADCC, half-life, and immune activation mechanisms.
Stable proteins ensure reliable assay performance over time and are essential for defining production and quality control parameters during drug manufacturing and regulatory submission.
Common modifications include addition of purification tags, biotinylation, fluorescent labeling, and fusion to carrier proteins or display systems such as VLPs.
Consistency is monitored through analytical testing that compares binding affinity, expression yield, and structural stability across different batches.