Highly-active GMP-grade CRISPR Cas9 protein
Due to the ease of design and operation, the CRISPR-Cas system has become the most widely used gene editing technology today. At KACTUS, we have developed a highly-active, wild-type Cas9 nuclease based on our SAMS™ technology platform. Our Cas9 nuclease has been codon-optimized and process-optimized to reach the best yield and purity. The addition of the nuclear localization signal (NLS) makes it suitable for gene editing work in eukaryotic cells.
Our GMP-grade, recombinant CRISPR Cas9 protein meets the highest quality and performance standards for gene editing research and manufacturing. Our GMP-grade Cas9 enzyme is manufactured in compliance with cGMP standards and accompanied by comprehensive quality documentation for regulatory support, making it eligible for CGT process development and CMC manufacturing. Finally, with a well-established global supply chain, we are capable of stably supplying our Cas9 enzyme in bulk quantities worldwide.
Why choose our Cas9 protein?
GMP Production
Our GMP-grade Cas9 is manufactured in compliance with GMP standards, ensuring the highest levels of quality and safety. We offer both research-grade and GMP-grade versions to seamlessly transition from preclinical study to clinical/commercial production.
Analytical Method Validation
We validate all analytical methods according to pharmacopeia standards, ensuring consistent performance and reliability of our CRISPR Cas9 protein. This rigorous validation process guarantees that our product meets the most demanding quality benchmarks in the industry.
Batch Consistency
Our rigorous quality control ensures every batch of our CRISPR Cas9 protein is manufactured with consistent quality and activity, providing you with a reliable product that delivers solid, consistent results every time. This consistency is critical for reproducible research outcomes and regulatory submissions.
High editing activity
Our CRISPR Cas9 protein demonstrates high editing activity, both in vitro and in vivo, enabling precise and efficient genome editing. This ensures that your gene editing experiments are both effective and reliable, reducing the need for extensive optimization.
QC & Stability Testing
We conduct comprehensive sterility and stability testing on our CRISPR Cas9 protein to ensure that it remains effective and safe over time. This confirms the protein’s longevity and reliability, even under varying storage and usage conditions. This documentation is also available to support regulatory filings.

Quality Documentation
We provide comprehensive quality documentation to support your IND and BLA filings, This includes animal-free origin statements (TSE/BSE), certificates of analysis, batch records, and other required documents to facilitate smooth regulatory submissions and approvals.
Product Performance Data
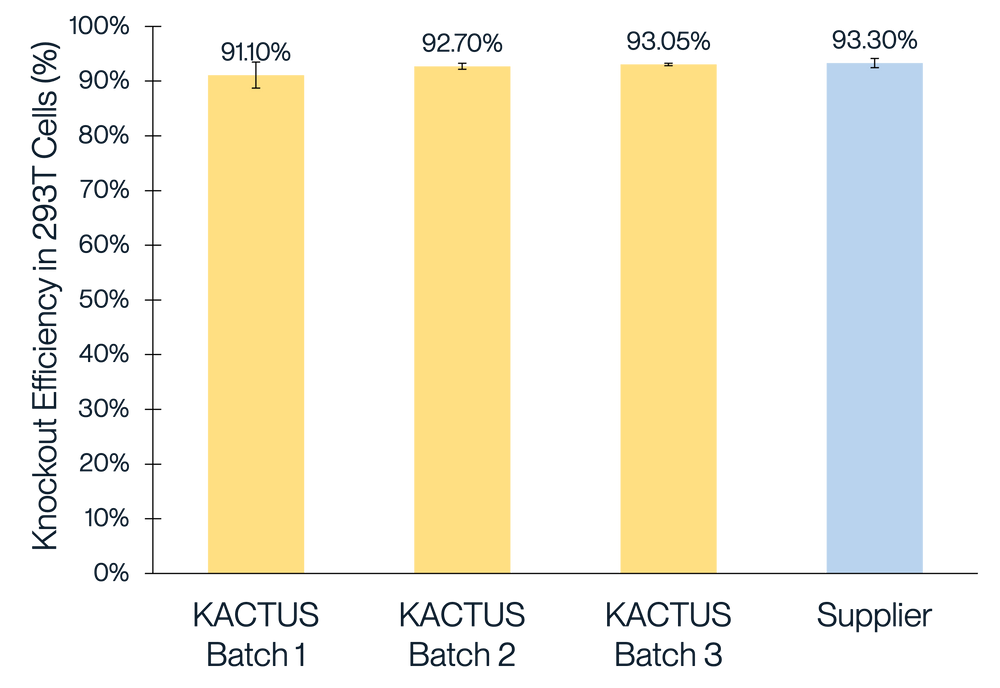
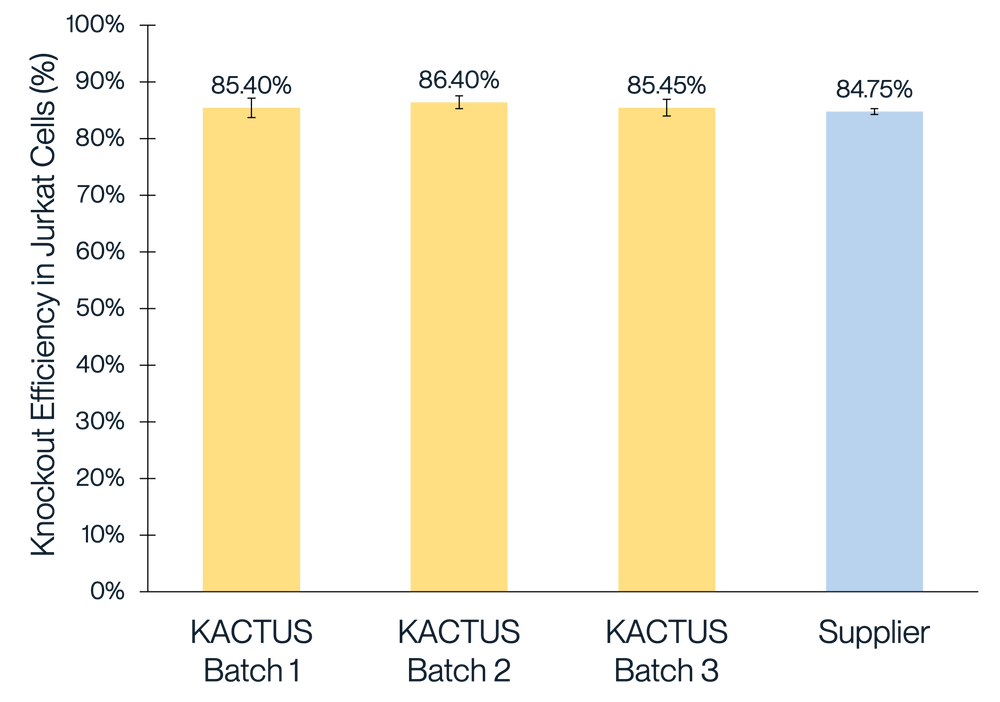
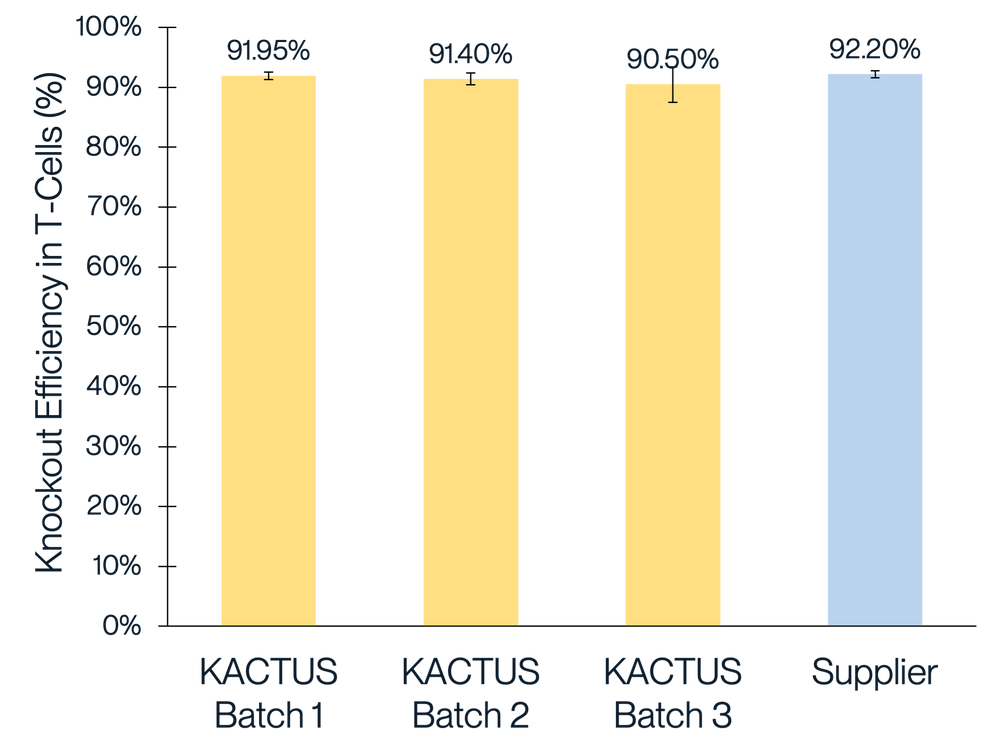
We've conducted extensive performance and analytical testing on our Cas9 protein to ensure high editing activity and quality. Our GMP-grade Cas9 protein demonstrates strong editing activity across various cell types, comparable to industry standards. We showcase successful gene knockouts in diverse cell lines, including 293T, Jurkat, and primary T cells, with editing efficacies greater than 85%. Our team has also conducted comprehensive stability testing on the Cas9 enzyme to underscore its reliability for use clinical and commercial applications.
High Editing Activity in Multiple Cell Types
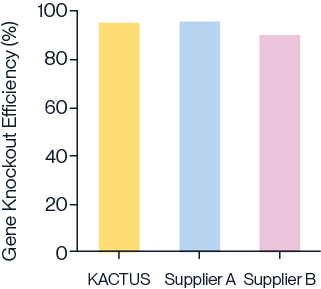
Figure 1. Gene knockout in 293T (A), Jurkat (B), and T cells (C) with different batches of KACTUS GMP-grade Cas9 protein. Results show greater than 85% editing efficacy across all three cell types, comparable to a leading supplier. Briefly, 75 pmol of Cas9 protein was electroported along with 225 pmol of sgRNA into various cells using the Lonza 4D Nucleofector system. The cells were cultured for three days followed by TIDE analysis. Gene knockouts: (A) knockout BCL11A in 293T cells; (B) knockout BCL11A in Jurkat cells; (C) knockout TRAC in Primary T cells.
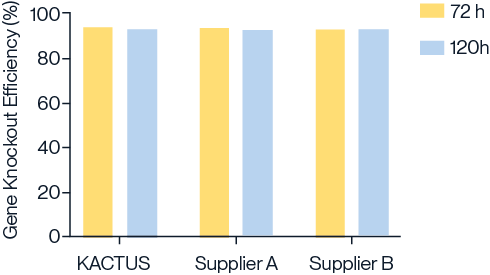
Comparable activity to leading suppliers
Figure 2. Comparison of gene knockout efficiency using Cas9 nucleases from KACTUS and two leading suppliers in UCAR-T cells. Gene knockout efficiency was assessed at 72 hours and 120 hours post-electroporation. Results indicate that KACTUS Cas9 nuclease performs comparably to leading suppliers, effectively knocking out genes related to immune rejection reactions on donor T cells.
Figure 3. Gene knockout efficiency of BCL11A in hematopoietic stem cells (HSCs) for the treatment of β-thalassemia using Cas9 nucleases from KACTUS and two leading suppliers. Results demonstrate that KACTUS Cas9 nuclease achieves gene knockout efficiency comparable to that of the leading suppliers, validating its effectiveness for therapeutic applications.
Batch consistency and long-term stability
Our rigorous quality control ensures every batch of our CRISPR Cas9 protein is manufactured with consistent quality and activity, providing you with a reliable product that delivers solid, consistent results every time. This consistency is critical for reproducible research outcomes and regulatory submissions.
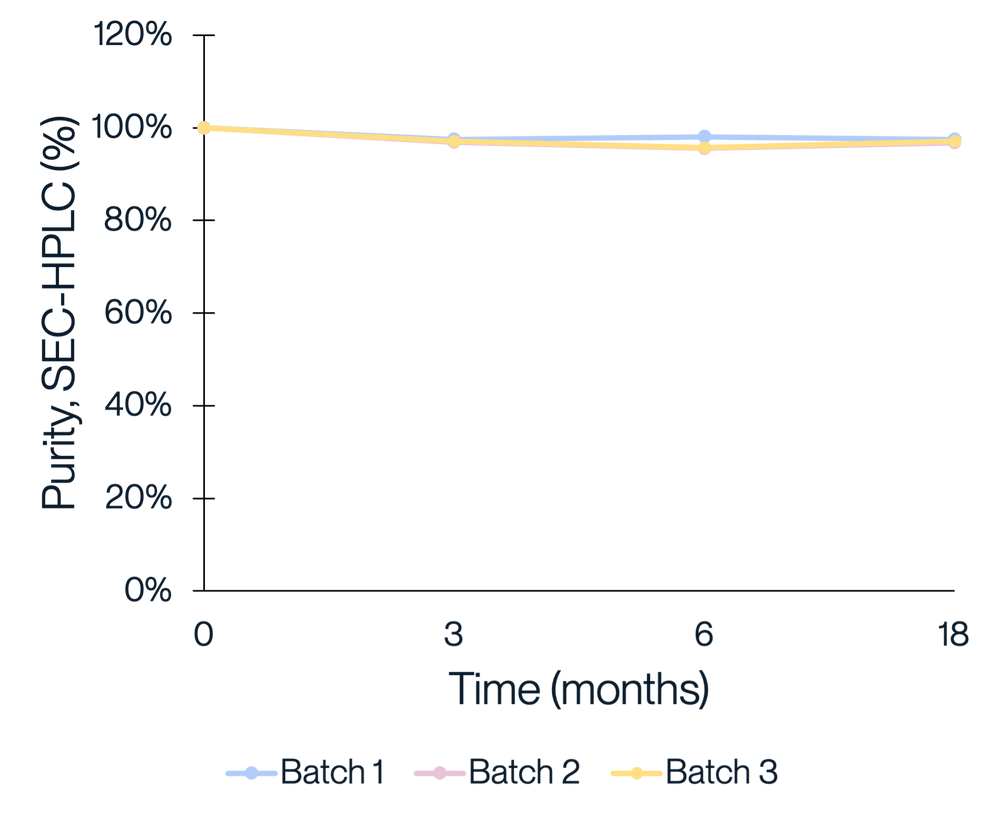
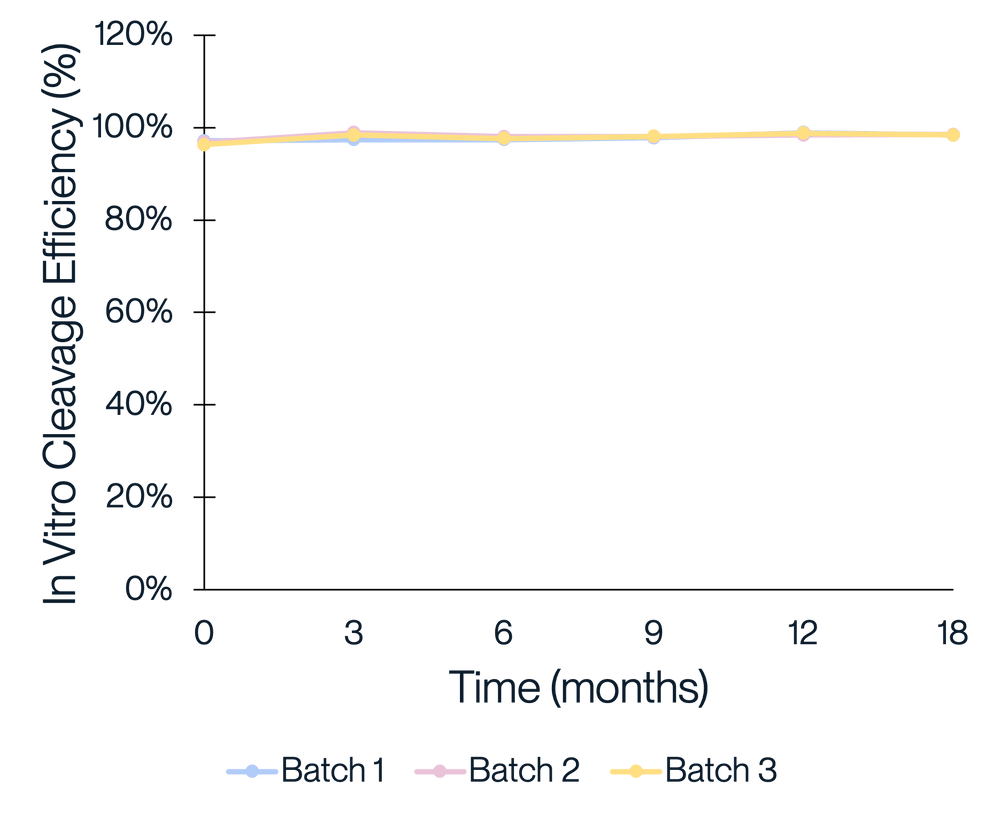
Figure 4. Consistent purity and activity performance was observed across three manufacturing batches of KACTUS GMP-grade Cas9. Three batches of KACTUS GMP-grade Cas9 were stored at -20°C for long-term stability testing. Purity and in vitro cleavage activity were analyzed at 3, 6, 9, 12, 15, 18 months, respectively. Results show our GMP-grade Cas9 has a good stability and consistent activity across time and different manufacturing batches.
Product Specifications & Quality
Product Specifications
Both our GMP-grade and research-grade Cas9 protein adhere to the following product specifications.
| Parameter | Specification |
|---|---|
| Express System | E. coli with CRISPR Cas9 gene of S. pyogenes |
| Concentration | 9.5-12.5 mg/mL |
| Form | Liquid |
| Storage Buffer | 30mM Tris-HCl, 0.3M NaCl, 50% Glycerol, 0.1 mM EDTA, pH 7.4 |
| Storage | -20±5°C for 18 months |
| Purity (Bis-Tris) | ≥ 95.0% |
| Purity (SEC-HPLC) | ≥ 95.0% |
| Activity | > 85.0% |
| Residual RNase | Negative |
Request a sample →
Quality Control Criteria
Each batch of our GMP-grade Cas9 Protein is tested for and meets the quality control criteria defined in the table below. Our research-grade Cas9 protein demonstrates similar performance but does not undergo lot-specific testing for all QC criteria.
| Assay | Acceptance Criteria | Appearance | Clear Liquid |
|---|---|
| Loading Amount | Not less than the amount of identification |
| Concentration | 9.5-11.5mg/mL |
| Identification | Corresponding to Reference Spectrum |
| Purity (RP-HPLC) | ≥ 95.0% |
| Purity (SEC-HPLC) | Monomer ≥ 95.0%, Aggregates ≤ 5.0% |
| Purity (NR-CE) | ≥ 85.0% |
| Purity (R-CE) | ≥ 90.0% |
| Bacterial Endotoxin | ≤ 10.0 EU/mg, (LOQ=0.1EU/mL) |
| Activity | ≥ 85.0% |
| Residual DNase | Negative (LOD=0.33U/μL) |
| Residual RNase | Negative (LOD=2.2×10⁻⁷U/μL) |
| Residual Host Cell Protein | ≤ 100.0 ng/mL (LOQ=3ng/mL) |
| Residual Host Cell DNA | ≤ 3.0 ng/mL (LOQ=0.03ng/mL) |
| Sterility | No growth |
| Residual Nickel Salt | ≤ 10.0 ppm (LOD=10.0ppm) |
| pH | 7.4±0.5 |
GMP-Grade Production
Our GMP-Grade Cas9 Protein is produced following cGMP standards, ensuring the traceability of raw materials, manufacturing process and QC release. This Cas9 protein is available in long-term bulk supply with batch-to-batch consistency to ensure suitability for industrial applications.
Documentation Package
Cas9 nuclease, GMP-grade comes with customizable regulatory documentation including Data Sheet, MSDS, COA, TSE/BSE statement, CoO. KACTUS has an integrative documentation system consisting of a digital quality management system, specification program, criteria support documents, and quality reports. Our product release process involves 15+ contaminant detection steps, 30+ quality tests, 4+ report reviews, and 4+ product release checkpoints. The documentation package is customizable based on relevant regulatory filings.
Quality Management System
→ ISO13485 Accreditation
→ Digital Manufacturing Execution System (MES)
→ Process and Analytical method validation
→ Batch-to-batch stability and consistency
→ Free from antibiotic residues and raw materials of animal origin
→ Comprehensive records for batch production
→ Pharmaceutical Class A & C Clean Room
→ Validated and maintained equipment
Universal Cas9 Protein ELISA kit
In ex vivo gene therapy, after cells are edited, the residual level of Cas9 protein must be measured before being reinfused into the patients. KACTUS has carefully developed a highly sensitive residual detection kit, the Cas9 (CRISPR Associated Protein 9) ELISA Kit. Our Cas9 ELISA Kit has also been validated with other leading commercial vendors and performs well as a universal Cas9 protein detection kit. The kit uses monoclonal antibodies in a sandwich ELISA and demonstrates high accuracy.
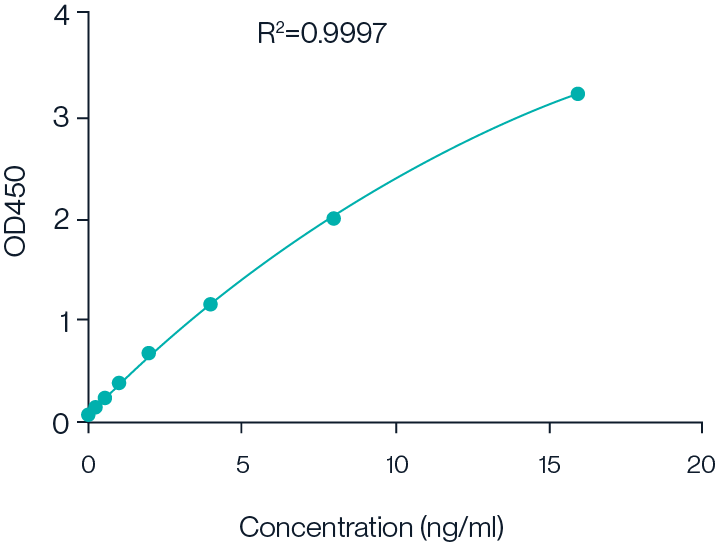
Figure 5. Example 8-point standard curve for Cas9 Protein ELISA kit.
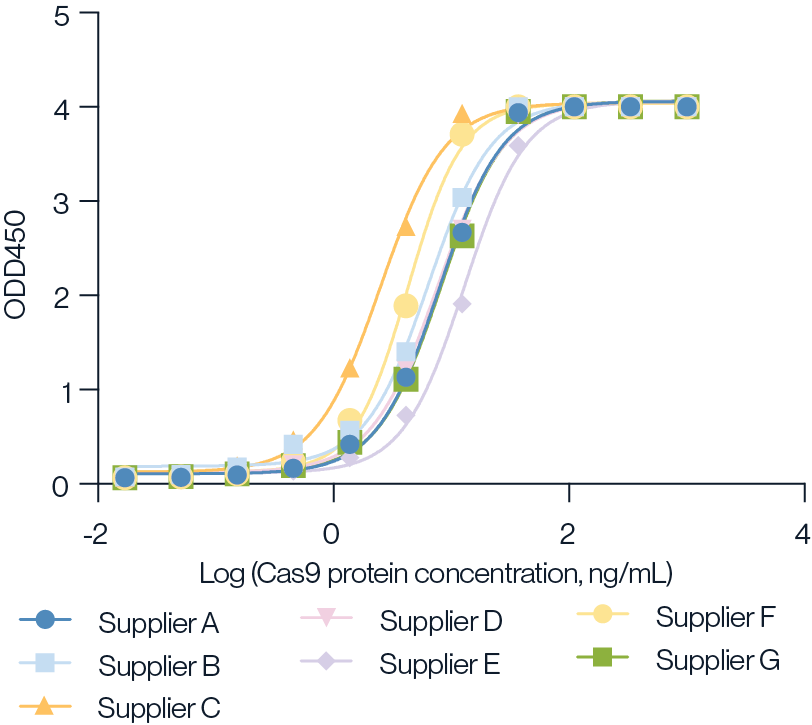
Figure 6. Detection of various suppliers Cas9 protein using KACTUS Cas9 ELISA Kit. The Cas9 products were serially diluted 3-fold from 1000ng/mL and tested following the KACTUS Cas9 ELISA kit procedure. Results show that the KACTUS Cas9 ELISA kit can be used for the quantification of various vendors' Cas9 protein. The sensitivity for other vendors was 0.2-0.4ng/mL, which is close to the sensitivity for KACTUS Cas9 protein (0.125ng/mL).
Explore Cas9 Products & Resources
Research-Grade Cas9 Protein
View our research-grade CRISPR Cas9 protein product page.
Cas9 ELISA Kit
View Cas9 Protein ELISA Kit product page to learn more or place an order.
Product Brochure
Access detailed information on our gene editing enzymes to see how they may support your research.
Research Poster
Explore our the use of our high-performing GMP Cas9 protein for cell and gene therapy applications.
CRISPR Cas9 Protein FAQs
KACTUS Cas9 is derived from Streptococcus pyogenes and includes codon optimization and a nuclear localization signal (NLS) for efficient delivery into eukaryotic cells. This structure enhances genome editing precision across mammalian systems.
The Cas9 protein is produced using a process-optimized E. coli expression system, which supports high yield, batch consistency, and scalability for both research-grade and GMP-grade applications.
The Cas9 protein has a molecular weight of approximately 163 kilodaltons (kDa) and is supplied at concentrations between 9.5 and 12.5 mg/mL in a liquid formulation.
Cas9 is formulated in 30 mM Tris-HCl, 0.3 M NaCl, 50 percent glycerol, and 0.1 mM EDTA at pH 7.4. It should be stored at minus 20 degrees Celsius with validated stability for at least 18 months.
KACTUS Cas9 exceeds 95% purity, confirmed via Bis-Tris SDS-PAGE and SEC-HPLC. Monomeric profiling ensures low aggregation levels, critical for genome editing applications.
Functional activity is assessed using in vitro DNA cleavage assays which quantify the proportion of target DNA successfully cleaved by the Cas9-sgRNA complex, indicating nuclease efficiency
When electroporated with sgRNA, KACTUS Cas9 demonstrates editing efficiencies greater than 85 percent in several tested target genes in human 293T cells, Jurkat cells, and primary T cells. Editing efficiency will vary depending on the target gene.
Each batch is tested for many parameters including RNase, DNase, host cell protein, host cell DNA, endotoxin, sterility, and residual nickel ions, with defined upper limits to ensure compliance with GMP standards.
KACTUS conducts long-term stability studies and batch-to-batch comparison testing, monitoring both purity and in vitro activity across multiple time points and manufacturing lots.
Yes. GMP-grade Cas9 is suitable for ex vivo gene editing in cell therapy manufacturing. KACTUS also offers a validated ELISA kit to detect residual Cas9 protein before cell reinfusion, supporting downstream QC compliance.
Request a test sample:
Related Products & Information
CRISPR Cas9 Protein
AccuBase® Base Editor
Cas9 News & Insights
Cas9 Enzyme Supports New IND Approval
Panorama of CRISPR Gene Editing Clinical Applications: 2024 Review
2024 Milestones in CRISPR Clinical Pipelines
IND Approval for Solid Tumor Therapy Using KACTUS Cas9 Enzyme
Gene Editing Drugs from the EMA Review Perspective
FDA New Guidelines Pave the Way for Gene Editing Drug Development
Exploring RNP: A Cutting-Edge Tool for Gene Delivery
Empowering Gene Editing Therapy: The Atlas of Gene Editing Enzymes from KACTUS
Gene Editing News & Insights
NK Cell Therapy Gets IND FDA Clearance with AccuBase® Base Editor from KACTUS
AccuBase™ Base Editor Continues to Advance NK Cell Therapies
Base Editing Technology Leads a New Chapter in Drug Development
Global Release of the First Commercial Base Editor on the Market: AccuBase™