Engineered base editor with near-zero off-target effects
AccuBase® is a cytosine base editor that converts a C•G base pair into a T•A base pair in the genome. It is independently designed by Base Therapeutics and manufactured for global sales by KACTUS. It creatively embeds the deaminase inside the Cas protein to prevent random tethering of deaminase to non-target site, significantly reducing off-target occurrence while still maintaining high editing efficiency.
Leveraging our SAMS™ protein engineering and expression platform, we have developed a large-scale production process for AccuBase® base editor. We have focused on maximizing the stability, purity, and activity of our GMP-grade AccuBase®, ensuring it meets the highest quality standards in compliance with cGMP raw materials for CGT drug manufacturing.
How does AccuBase® work?
Precision editing by activating only upon accurate target binding
After forming a ribonucleoprotein (RNP) with sgRNA, the AccuBase® protein remains in a non-editing state before binding to the target DNA. The deaminase is encapsulated inside the Cas9n protein and does not interact with any non-target DNA, thus significantly reduces the risk of off-target effects. When the RNP binds to the target DNA, the conformation of AccuBase® changes, leading to the exposure of the deaminase domain and effectively editing bases within the 3-12 window range of the target site (with the first position being the farthest from the PAM) (Figure 1).
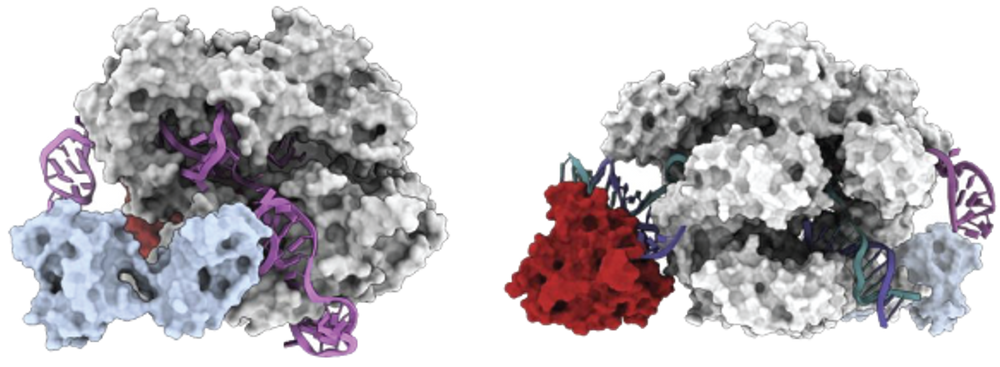
Figure 1. AccuBase® in the non-editing stage (left) and editing stage (right).
Mechanism of action
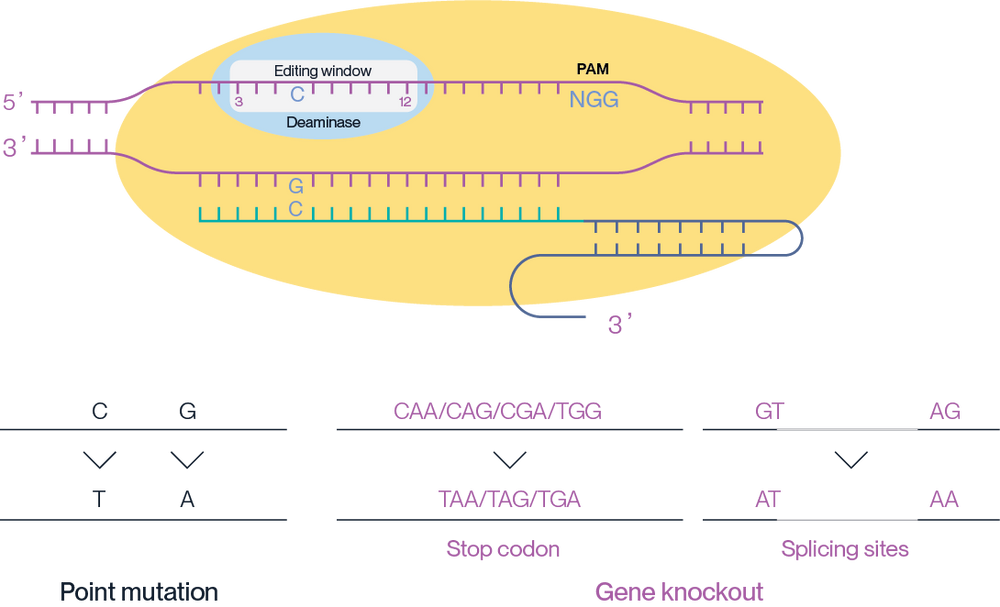
The mechanism of action of AccuBase® involves the precise editing of target DNA sequences within the 3-12 base window of the target site, leading to specific point mutations (C to T, G to A) or gene knockouts through the introduction of stop codons or alteration of splicing sites (Figure 2).
Product Performance Data
Near-zero off-target effects
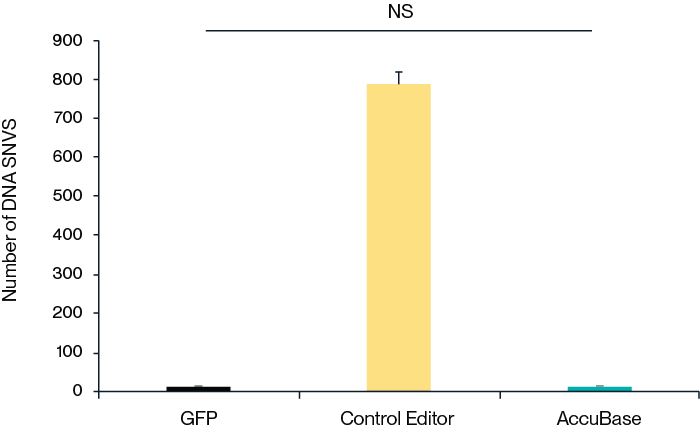
Figure 3. Measurement of off-target effects by GOTI. By leveraging the GOTI (genome-wide off-target analysis by two-cell embryo injection) to measure the off-target effects throughout the whole genome, it was shown that compared to the control base editor (with 700 SNVs detected), the number of SNV obtained after editing by AccuBase® is similar to the GFP (negative control) group, suggesting a near-to-zero off-target effects of AccuBase®.
High editing activity
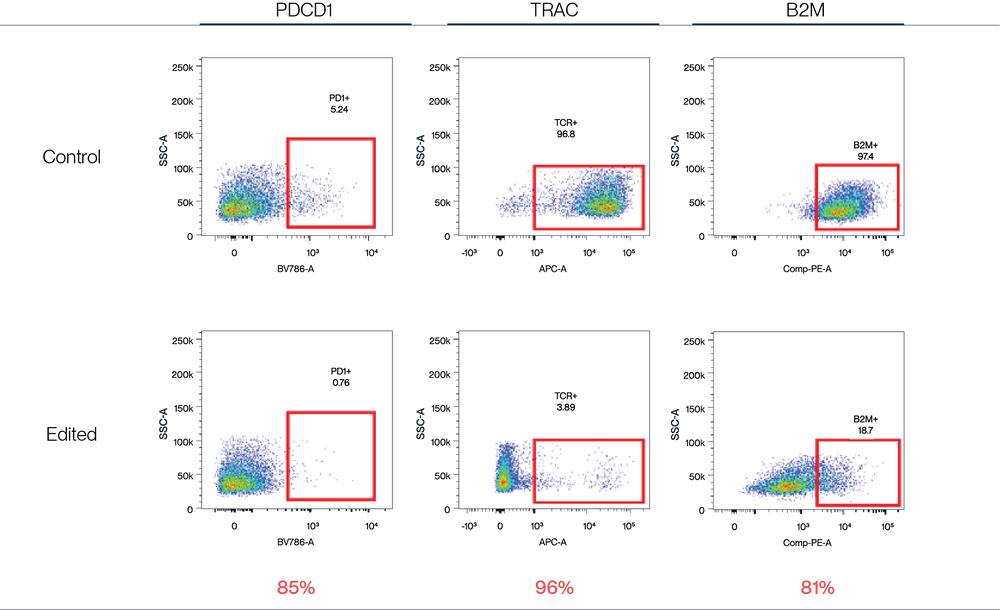
Figure 4. AccuBase™ RNP was electroporated into activated primary T cells. According to flow cytometry, AccuBase™ can efficiently knock out PD1, B2M, and TRAC proteins on the membrane of activated primary T cells at the protein level. For PD1 and B2M, the knockout efficiency exceeded 80%, while the knockout efficiency of TRAC reached 96%. FACS efficacy % = (positive control - KO group)/positive control* 100%.
Avoids INDEL creation
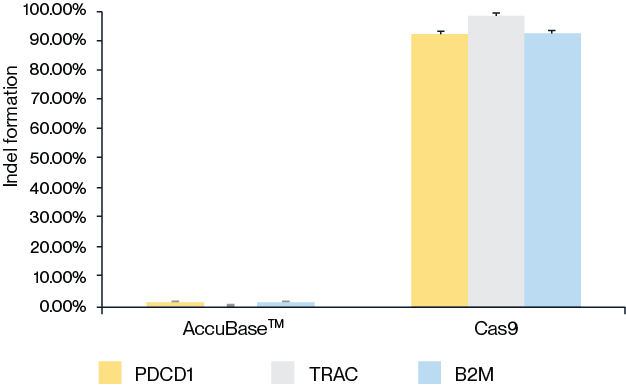
Figure 5. Compared with Cas9 protein, AccuBase™ does not produce DNA horizontal insertions and deletions (INDELs).
Avoids chromosomal translocation
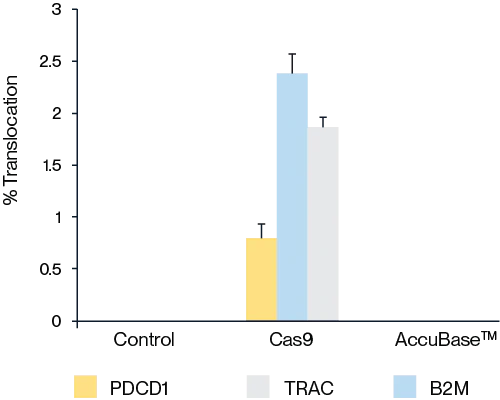
Figure 6. Compared with Cas9 protein, AccuBase® does not cause chromosomal translocation.
Product Features
KACTUS offers both GMP-grade and research-grade AccuBase® as off-the-shelf catalog products. Our GMP AccuBase® Base Editor is free from animal-derived components, has greater than 80% purity, and comes with comprehensive quality control documentation as well as additional batch production records upon request. We are dedicated to supporting your applications of AccuBase® from preclinical research through commercial manufacturing, backed by our robust technical and quality support.
Learn more about our GMP quality management systems →.
Manufactured in a GMP-compliant facility
Raw materials free from animal-derived components
High purity, activity, and stability

Traceable documentation for regulatory support
Off-the-shelf catalog product
Product Specifications & Quality
Product Specifications
Both our GMP-grade and research-grade AccuBase® adhere to the following product specifications:
| Parameter | Specification |
|---|---|
| Express System | E. Coli |
| Concentration | 10mg/ml |
| Molecular Weight | 210.14kDa |
| Form | Liquid |
| Storage Buffer | 30 mM Tris, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 50% Glycerol, pH8.0 |
| Storage | Store at -80 ±10°C |
Request a sample →
Quality Control Criteria
Our GMP-grade AccuBase® meets the following quality control criteria:
| Assay | Acceptance Criteria |
|---|---|
| pH | 8.0±0.5 |
| Concentration | 9.0-11.0mg/mL |
| Purity (electrophoresis) | ≥ 80.0% |
| Purity (RP-HPLC) | ≥ 88.0% |
| Purity (SEC-HPLC) | ≥ 80.0% |
| Residual DNase | Sample/Control ≤ 3.0 |
| Residual RNase | Sample/Control ≤ 3.0 |
| Residual Host Cell Protein | ≤ 100.0ng/mL |
| Residual Host Cell DNA | ≤ 200.0ng/mL |
| Endotoxin | ≤ 10.0EU/mg |
| Sterility | Negative |
| Mycoplasma | Negative |
GMP Compliance
GMP-Grade AccuBase® is produced following cGMP standards, ensuring traceability of raw materials, manufacturing processes, and QC release testing. The GMP-Grade AccuBase® is available in long-term bulk supply with high batch-to-batch consistency to ensure suitability for industrial applications. We also offer a research-grade version for a seamless transition from preclinical study to clinical/commercial CGT drug manufacturing.
GMP-Grade AccuBase® comes with customizable regulatory documentation including Data Sheet, MSDS, COA, TSE/BSE statement, CoO. KACTUS has an integrative documentation system consisting of a digital quality management system, specification program, criteria support documents, and quality reports. The documentation package is customizable based on relevant regulatory filings.
→ ISO13485 Accreditation
→ Digital Manufacturing Execution System (MES)
→ Process and Analytical method validation
→ Batch-to-batch stability and consistency
→ Free from antibiotic residues and raw materials of animal origin
→ Comprehensive records for batch production
→ Pharmaceutical Class A & C Clean Room
→ Validated and maintained equipment
AccuBase® ELISA kit
We have developed an AccuBase® ELISA kit for detection of residual AccuBase® in samples. The kit uses monoclonal antibodies in a sandwich ELISA and demonstrates high accuracy.
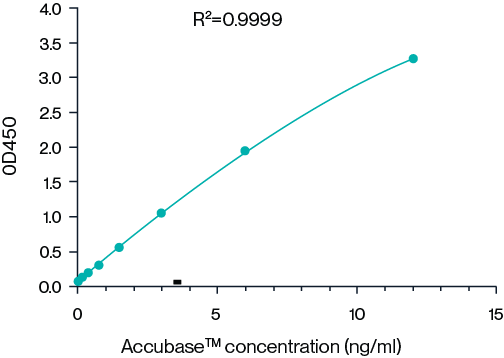
Figure 7. Example 8-point standard curve for AccuBase® ELISA kit. The quantitative range of this ELISA kit is 187.5 pg/mL - 12 ng/mL.
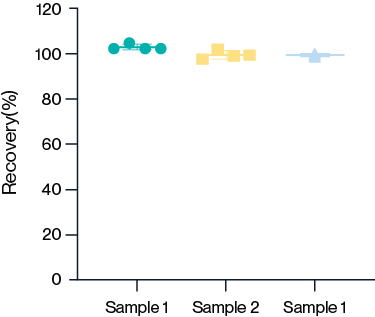
Figure 8. The recovery rate of the kit was analyzed by testing three AccuBase® samples with known concentrations in dilution buffer using the same AccuBase® ELISA kit. Results show that the recovery rate of various sample concentrations is between 80% and 120%, indicating high accuracy.
Frequently Asked Questions
Product Specifications
- No double-strand DNA breaks. Compared to CRISPR/Cas9, AccuBase® does not cause DNA double-strand breaks, eliminating risks of DNA INDELs and chromosomal translocations.
- Near-zero off-target effects. Compared to the previous generation of Cytosine Base Editors (CBEs), AccuBase®'s unique structural design releases deaminase activity only at the editing site, offering near-zero off-target effects and enhanced safety while maintaining high editing efficiency.
- GMP-grade and Research-Grade. Suitable for preclinical work, process development, and clinical research applications.
- Independent intellectual property and FTO available. It has completed independent intellectual property rights and holds foundational technology patents, with FTO (Freedom to Operate) designation.
AccuBase® is an engineered DNA cytosine base editing protein that integrates a deaminase within the Cas9 nickase protein. When the AccuBase® protein forms a ribonucleoprotein (RNP) complex with single guide RNA (sgRNA), it remains in a non-editing state until it binds to the target DNA. Upon binding, a conformational change exposes the deaminase domain, enabling precise C-to-T editing within a 3-12 nucleotide window (with the first position being the farthest from the PAM sequence). In summary, AccuBase® significantly reduces random off-target risks while maintaining high-efficiency on-target editing.
The research-grade and GMP-grade products utilize the same bacterial source, raw materials, and production process, resulting in consistent protein activity. The differences are:
- Production environments: GMP-grade is produced in a cGMP environment, whereas research-grade is produced in non-GMP conditions.
- Quality control testing: GMP-grade AccuBase® undergoes comprehensive QC testing, including appearance, concentration, purity (2100), purity (SEC-HPLC), purity (RP-HPLC), endotoxin, residue DNase and RNase, host protein and DNA residue, sterility, and mycoplasma. Research-grade AccuBase® QC testing includes concentration, identification (Bis-Tris PAGE), purity (SEC-HPLC), and endotoxin.
- Traceability documentation: GMP-grade has a full set of validation reports, stability study, COA, COO, animal-free statement, etc., and supports on-site audits.
The molecular weight of AccuBase® is 210.14 kDa. The conversion is 10 mg/mL = 10 μg/μL = 47.59 μM; 1 μg = 4.759 pmol.
30 mM Tris, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 50% Glycerol, pH 8.0.
-80±10℃ for 3 years, avoid to repeated freeze-thaw.
Product Usage
- Correcting a single point mutation to restore the gene function
- Gene knockout by introducing a stop codon or altering splice sites, to silence a gene expression.
- Multi-locus gene editing. It can also be combined with other gene editing tools.
- Electroporation or direct injection into cells, including adherent cells, suspension cells, embryonic cells (fish, mice, etc.), and plant embryos (gene gun method).
AccuBase® can not only achieve single-gene knockout but also simultaneously knock out 2, 3, or more genes.
The sgRNA scaffold used in AccuBase® is identical to that of spCas9. However, when designing sgRNAs for gene knockout applications, it is crucial to ensure that the target site includes editable cytosines (C) within the 3-12 nucleotide window. This positioning is essential to achieve efficient base editing while maintaining high specificity.
Using human TRAC gene as an example:
- Download the gene sequence with annotated information and open it using SnapGene software.
- Manually search the sequence information for the codon CAA, CAG, CGA, or TGG.
- Check nearby for a suitable PAM sequence, using the NGG PAM sequence. Ensure that the edited C is within the editing window (3-12). Similar conditions apply to CAG and CGA. If it's TGG, check the position of the PAM on the antisense strand.
AccuBase® is a next-generation DNA cytosine base editing protein designed and developed by Base Therapeutics. It has completed independent intellectual property rights and holds foundational technology patents, with FTO (Freedom to Operate) available. Base Therapeutics is committed to an open, flexible, and sincere approach in its friendly negotiations with potential partners/clients.
Please find the usage details in the datasheet. For primary NK cells and primary T cells editing, in a 20μL electroporation system using Lonza's nucleofection device, the recommended amount of AccuBase® for electroporating 1E+06 cells is 80 pmol, with a 1:1 molar ratio to sgRNA. The recommended electroporation program is CM156.
For gene level analysis: Use Sanger sequencing followed by analysis with the online software EditR.
For protein level analysis: Use FACS flow cytometry (for cell membrane proteins).
Ordering Information
You can order AccuBase® directly online via credit card or PO. Please send PO information to orders@kactusbio.us. To request a quote, please contact sales@kactusbio.us.
Research-grade: 100ug/vial, 200μg/vial, 500μL/vial, and 1mg/vial, at 10mg/mL concentration.
GMP-grade: 1mg/vial at 10mg/mL concentration
Bulk sizes are available. Please contact sales@kactusbio.us.
AccuBase®, both research-grade and GMP-grade, is an off-the-shelf product. Catalog sizes ship
next-day in the contiguous US. For international customers, please expect up to a one-week lead time. For the lead time and price of bulk orders, please contact sales@kactusbio.us.
Explore AccuBase® Products & Resources
Research-Grade AccuBase®
View our research-grade AccuBase® base editor product page.
AccuBase® ELISA Kit
View AccuBase® ELISA Kit product page to learn more or place an order.
Product Brochure
Access detailed information on AccuBase® to see how it may support your research.
Research Poster
Explore the use of AccuBase® for editing multiple genes in primary human T cells.
AccuBase® Cytosine Base Editor FAQs
AccuBase® is a cytosine base editor engineered to convert C•G into T•A base pairs with high specificity. Unlike traditional base editors, AccuBase® integrates the deaminase domain within the Cas protein structure, minimizing exposure and reducing off-target activity.
AccuBase® is available in a GMP-grade format, produced under strict quality control standards. It uses animal-free components, is manufactured in line with regulatory guidelines, and comes with comprehensive batch documentation, making it suitable for CGT manufacturing workflows.
AccuBase® remains in a non-editing state until it binds to its target DNA. Its unique structural design conceals the deaminase, exposing it only when bound to the correct site—this significantly reduces off-target interactions with non-target DNA sequences.
AccuBase® enables precise C-to-T and G-to-A transitions, allowing for:
– Introduction of premature stop codons
– Splice site disruption
– Gene knockout and exon skipping applications
AccuBase® has been validated using GOTI to confirm minimal off-target activity. Flow cytometry shows knockout rates above 80 percent for PD1 and B2M, and 96 percent for TRAC in primary T cells.
No. Unlike traditional Cas9 nucleases, AccuBase® does not introduce INDELs or chromosomal translocations, offering a safer genome editing alternative with cleaner outcomes.
AccuBase® is produced in E. coli and supplied as a liquid formulation (10 mg/mL) in a stabilizing buffer. It should be stored at –80°C for long-term stability.
AccuBase® is available in both research-grade and GMP-grade formats:
– Research-grade: High-purity, validated for performance
– GMP-grade: Includes full regulatory documentation, QA release records, and enhanced process traceability.
QC metrics include endotoxin levels below 10 EU per mg, residual host DNA under 200 ng per mL, and greater than 80 percent purity by electrophoresis, HPLC, and SEC-HPLC.
Yes. Both research-grade and GMP-grade AccuBase® samples are available. You can also request batch records, QC reports, and other technical documentation to support your CGT research or manufacturing workflow.
Request a test sample:
Related Products & Information
AccuBase® Base Editor
CRISPR Cas9 Protein
AccuBase® News & Insights
NK Cell Therapy Gets IND FDA Clearance with AccuBase® Base Editor from KACTUS
AccuBase™ Base Editor Continues to Advance NK Cell Therapies
Base Editing Technology Leads a New Chapter in Drug Development
Global Release of the First Commercial Base Editor on the Market: AccuBase™
Gene Editing News & Insights
KACTUS' Cas9 Enzyme Powers IND-Approved Cell Therapy
Panorama of CRISPR Gene Editing Clinical Applications: 2024 Review
2024 Milestones in CRISPR Clinical Pipelines
IND Approval for Solid Tumor Therapy Using KACTUS Cas9 Enzyme
Gene Editing Drugs from the EMA Review Perspective
FDA New Guidelines Pave the Way for Gene Editing Drug Development