STEAP1, the next blockbuster target for Prostate Cancer
By Tingxu Chen
Prostate cancer (PCa) is the second most common cancer among men, with a mortality rate of approximately 2.3%. According to the American Cancer Society, around 35,000 men die of prostate cancer each year in the United States, and nearly 400,000 deaths occur globally[1], with most deaths occurring in men over the age of 75.
Among all PCa subtypes, metastatic castration-resistant prostate cancer (mCRPC) is the most challenging to treat, with a median survival time of less than three years. Currently, the prostate-specific membrane antigen (PSMA) is the predominant diagnostic and therapeutic target for mCRPC, used in imaging and treatments such as radionuclide therapy (e.g., [^177Lu]Lu-PSMA Pluvicto). However, issues such as drug resistance, disease recurrence, and low PSMA expression in part of the patient populations also limit its efficacy, which highlights the urgent need for discovering alternative therapeutic targets.
The STEAP (Six Transmembrane Epithelial Antigen of the Prostate) protein family is a group of proteins that was first discovered in 1999. Within this protein family, STEAP1 was identified as a prostate-specific, highly expressed transmembrane protein with minimal expression level in normal tissues. Studies over the past two decades have consistently shown that STEAP1 is closely associated with PCa progression—including bone metastasis and castration resistance—and that its expression level positively correlates with patients with poor prognosis, which makes STEAP1 a promising target for PCa diagnosis and treatment. At the 2024 AACR Annual Meeting, Amgen presented the promising Phase 1 clinical data for AMG509 (Xaluritamig), a T-cell engager (TCE) targeting STEAP1. In patients with mCRPC, 49% experienced a ≥50% reduction in PSA levels, and 28.4% achieved a ≥90% reduction. The objective response rate (ORR) in the high-dose group was 41%, with a disease control rate (DCR) of 79%. This marks the first STEAP1-targeted therapy to demonstrate such high efficacy in advanced prostate cancer, solidifying STEAP1 as a hot target in this space.
STEAP1 is a six-transmembrane protein with both its N- and C-termini located in the cytoplasm. Unlike other STEAP family members, STEAP1 lacks an NADPH-binding FNO domain in its N-terminus and cannot independently catalyze Fe³⁺ or Cu²⁺ reduction 222, requiring interaction with other STEAP proteins[2]. A truncated isoform, STEAP1B, is considered to be involved in metal ion metabolism and the regulation of malignant tumor phenotypes.
The structure of STEAP1; STEAP1 is highly expressed in prostate cancer tissues [2]
STEAP1 is predominantly expressed at cell–cell junctions, especially in prostate secretory epithelial cells, maintaining cellular redox balance and regulating proinflammatory cytokine signaling. In cancers such as prostate and bladder cancer, STEAP1 is highly expressed and critical for tumor proliferation and metastasis. In mCRPC, the STEAP1 expression is driven by androgen receptor (AR) signaling. Even under low-testosterone, castration-resistant conditions, AR splice variants (e.g., AR-V7) can continue to activate STEAP1 to maintain cancer cell survival. Overexpression of STEAP1 suppresses CD8⁺ T cell infiltration, enhances immunosuppressive microenvironment, and accelerates disease progression. It has also been shown that STEAP1 contributes to radiotherapy and chemotherapy resistance, probably via mechanisms involving ferroptosis inhibition.
Mechanism of action of STEAP1 [3]
Advances in STEAP1-Targeted Therapies
Currently, the drug development targeting STEAP1 is under rapid progressing, with antibody-drug conjugates (ADCs) and T-cell engagers (TCEs) emerging as the leading modalities. Both single- and dual-antigen targeting strategies are being explored, primarily for mCRPC, as well as in other solid tumors.
ADCs:
Adcentrx’s ADRX-0405 employs a high drug-to-antibody ratio (DAR = 8), protease-cleavable linkers, and stable conjugation chemistry to enhance payload delivery and ADC stability.
DAC Biotechnolog’s DXC008 and AbbVie’s ABBV-969 are dual-targeting ADCs aimed at both STEAP1 and PSMA, designed to overcome drug resistance and improve efficacy. Both candidates are currently in Phase 1 trials.
TCEs:
Amgen’s AMG509 (Xaluritamig) is a leading STEAP1-targeted TCE, utilizing a 2+1 bispecific format to bind two STEAP1 molecules and one CD3 molecule. The clinical trial has proceeded directly from Phase 1 to Phase 3, reflecting its strong clinical and commercialization potential [4].
BC261, developed by Memorial Sloan Kettering Cancer Center, uses a 2+2 IgG-[L]-scFv structure targeting a conserved extracellular domain of STEAP1. This format may offer more consistent binding and efficacy across species. BC261 is currently in preclinical development [5].
Diagnostic Imaging and Surgical Applications
Beyond therapeutics, STEAP1 also holds promise for clinical imaging and precision surgery. In a recent study, scientists developed a fluorescent imaging probe using extracellular vesicles engineered to target STEAP1. This probe significantly improved the imaging accuracy and contrast, reducing the positive surgical margin rate from 100% to 0% in murine models. It also extended postoperative survival, offering a valuable reference for precision-guided prostate cancer surgery [7].
List of therapeutic drugs targeting STEAP1 [2]
Structure of Amgen's TCE drug AMG-509 [4]
During AMG-509 treatment, the patient's serum PSA level decreased significantly [4]
Due to its tumor-specific high expression, significant role in therapy resistance, and favorable draggability, STEAP1 has become one of the most promising therapeutic targets in prostate cancer. Drugs targeting STEAP1 could fill critical gaps in the treatment landscape, particularly for patients resistant to AR inhibitors, and may emerge as the next major target after PSMA.
By leveraging the recombinant multi-transmembrane protein technology platform, KACTUS has developed a full-length, conformationally correct STEAP1 protein in VLP format with stable and robust biological activity, serving as a powerful tool for developing and validating STEAP1-targeted therapies.
Product Data:
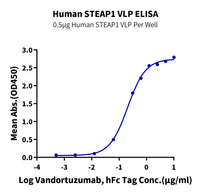
Immobilized Human STEAP1 VLP at 5 μg/ml (100 μl/well) on the plate. Dose response curve for Vandortuzumab with the EC 50 of 0.21 μg/ml determined by ELISA.
Product List:
|
Part Number |
Information |
|
Human STEAP1 Protein VLP |
References:
[1] Prostate Cancer: A Review of Genetics, Current Biomarkers and Personalized Treatments. doi: 10.1002/cnr2.70016.
[2] STEAP1–4 (Six-Transmembrane Epithelial Antigen of the Prostate 1–4) and Their Clinical Implications for Prostate Cancer. doi: 10.3390/cancers14164034.
[3] STEAP Proteins: Roles in disease biology and potential for therapeutic intervention. doi: 10.1016/j.ijbiomac.2025.142797.
[4] AMG 509 (Xaluritamig), an Anti-STEAP1 XmAb 2+1 T-cell Redirecting Immune Therapy with Avidity-Dependent Activity against Prostate Cancer. doi: 10.1158/2159-8290.CD-23-0984.
[5] Novel potent anti-STEAP1 bispecific antibody to redirect T cells for cancer immunotherapy. DOI: 10.1136/jitc-2021-003114.
[6] A novel prostate cancer-specific fluorescent probe based on extracellular vesicles targeting STEAP1 applied in fluorescence guided surgery. doi: 10.1016/j.jconrel.2025.01.079.
[7] Phase I Study of DSTP3086S, an Antibody-Drug Conjugate Targeting Six-Transmembrane Epithelial Antigen of Prostate 1, in Metastatic Castration-Resistant Prostate Cancer. doi: 10.1200/JCO.19.00646.
[8] The Usefulness of STEAP Proteins in Prostate Cancer Clinical Practice. doi: 10.36255/exonpublications.prostatecancer.steap.2021 (https://www.ncbi.nlm.nih.gov/books/NBK571332/)
[9] Clinical significance of STEAP1 extracellular vesicles in prostate cancer. doi: 10.1038/s41391-021-00319-2.
[10] Development of STEAP1 targeting chimeric antigen receptor for adoptive cell therapy against cancer. doi: 10.1016/j.omto.2022.06.007.
[11] The Usefulness of STEAP Proteins in Prostate Cancer Clinical Practice. DOI: 10.36255/exonpublications.prostatecancer.steap.2021
[12] Targeting STEAP1 as an anticancer strategy. doi: 10.3389/fonc.2023.1285661.
[13] Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. doi: 10.1038/s41467-023-37874-2.
[14] The Role of STEAP1 in Prostate Cancer: Implications for Diagnosis and Therapeutic Strategies. https://doi.org/10.3390/biomedicines13040794
[15] Vaccines as treatments for prostate cancer. doi: 10.1038/s41585-023-00739-w.