Ensuring Quality in Raw Material Enzymes
Raw material enzymes play a pivotal role in the manufacturing of mRNA and gene therapies. We offer large-scale GMP-grade recombinant enzymes with various size fermentation tanks, designed to provide yields tailored to your specific needs. We are committed to producing the highest quality enzymes that meet stringent GMP standards internationally.
Our strict compliance with cGMP regulations, combined with advanced and comprehensive analysis and testing equipment, ensures thorough quality release testing and the highest standards of enzyme reliability.
Overview of GMP Compliance
The safety and quality of raw and ancillary materials are critically important in preclinical, clinical, and commercial manufacturing. Our recombinant proteins are produced and evaluated in compliance with GMP (Good Manufacturing Practice) guidelines, following an ISO13485 certified quality management system, and using animal-origin free materials. To further ensure that our products do not affect your final product, we employ more rigorous quality control measures, which include sterility, endotoxin, heavy metal, residual DNase, etc.
Process Control & Optimization
Quality Management System
GMP Facility
5,000 m²
R&D, Production, Quality Control Center
10,000 m²
GMP-Grade Manufacturing Site
1,000 L
Fermentor for Production of GMP-Grade Enzymes
85%
Employees in R&D, Manufacturing, and Quality Control
Large-Scale Protein Expression & Purification Systems
Our Certifications

ISO13485 Certified
ISO13485:2016 certification is an internationally recognized standard that sets out the requirements for a quality management system that ensures companies adhere to stringent quality control measures, regulatory compliance, and risk management practices throughout the entire product lifecycle. Our company has obtained ISO13485:2016 certification to demonstrate our commitment to producing safe, reliable, and high-quality proteins and enzymes.
Approval ID: 00034327

FDA Drug Master Files (DMF)
A DMF (Drug Master File) is a document submitted by a company to the FDA. The document contains detailed information on the entire production process, including product formulations, plant facilities, production processes, and any other procedures involved in this product. DMF filing demonstrates transparency by the company and willingness to work alongside regulations. Additionally, when companies apply for a new product registration with the FDA, using materials with a DMF will shorten the registration time.
Comprehensive Product Release Process
→ 01
Quality inspection tasks of control sample
Concentration, Identity, Endotoxin
→ 02
Issue central control sample report
Review & approve report
→ 03
Quality inspection tasks for raw material
Concentration, Identity, Purity, Endotoxin, HCD Residue, HCP Residue
→ 04
Issue raw material product report
Review & approve report
→ 05
Dilute product stock solution
Semi-finished product
→ 06
Quality inspection tasks of diluted product
Concentration
→ 07
Issue semi-finished product report
Review & approve report
→ 08
Aliquot finished product
→ 09
Issue finished product report
Review & approve report
→ 10
Release finished product
→ 11
Ongoing stability testing
Long-term & accelerated stability testing
End-to-end supply chain management for reliable, long-term delivery of your enzyme.
Inventory Hub
Our inventory hub located in Waltham, MA allows for centralized distribution of our products to customers globally.
Distribution Network
Our comprehensive network of distributors creates seamless ordering within your country, simplifying the customs and import process.
Robust GMP Facility
Our 10,000m² cGMP manufacturing facility boasts various fermentation tanks up to 1000L for long-term large-scale raw material supply.
Seamless Transition from Research to GMP
Utilize our affordable research-grade enzymes during preclinical stages to simplify your process development efforts. With the same purity and performance as GMP-Grade, you will be able to seamlessly transition to clinical manufacturing, while ensuring stability, safety, potency, and purity in your final biologics.
Custom GMP Enzyme Production
Looking to accelerate your research with reliable, high-quality enzymes? Our custom GMP-grade enzyme production service is tailored to meet your specific needs, providing you with top-tier enzymes for your clinical and commercial manufacturing. Whether you require bulk quantities or hard-to-find enzymes, we've got you covered.
Stability Testing
Our stability research includes high temperature test, light test, repeated freeze-thaw test, simulated transportation test, accelerated stability test, inter-batch stability study, long-term stability study, etc., to ensure the stability of the product in an all-around way.
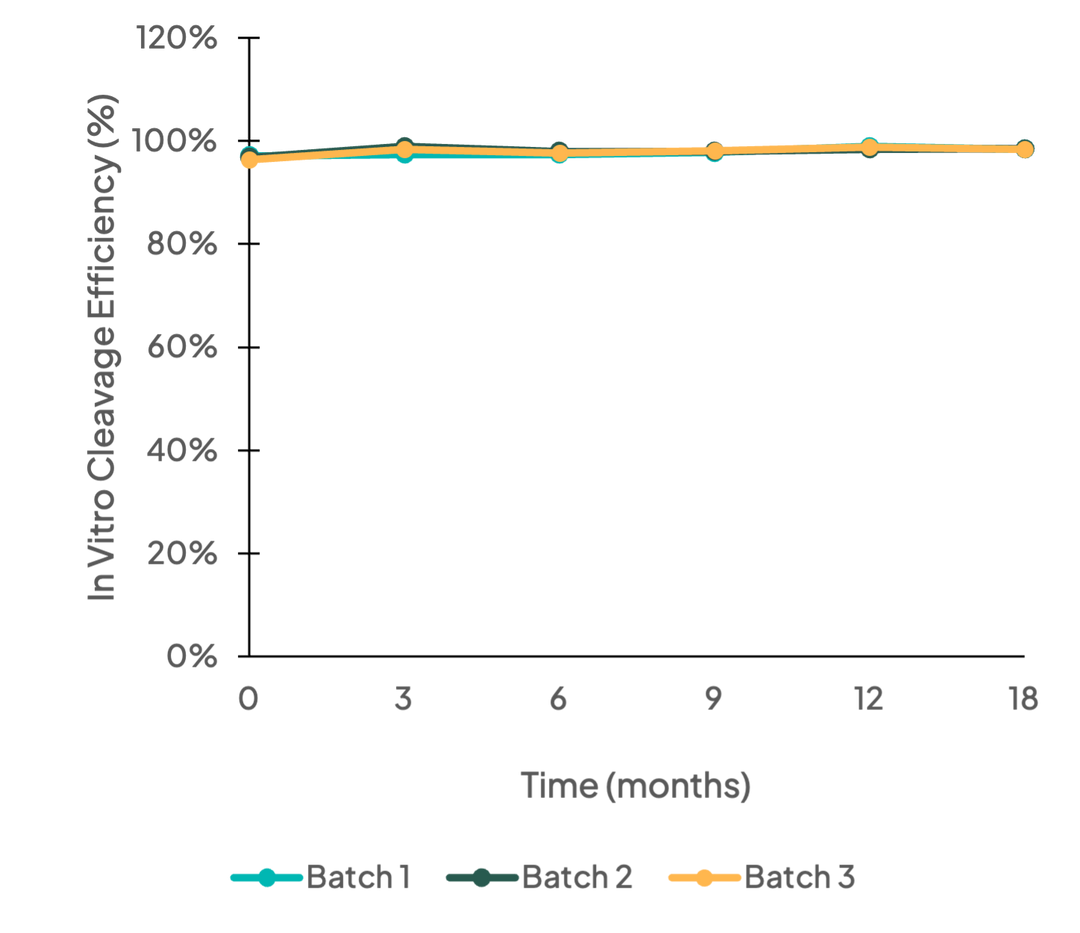
Activity
Long-term stability testing (0-18 months) of three batches of Cas9 nuclease was performed using in vitro cleavage activity. Results demonstrate Cas9 nuclease has good long-term stability and batch-to-batch consistency.
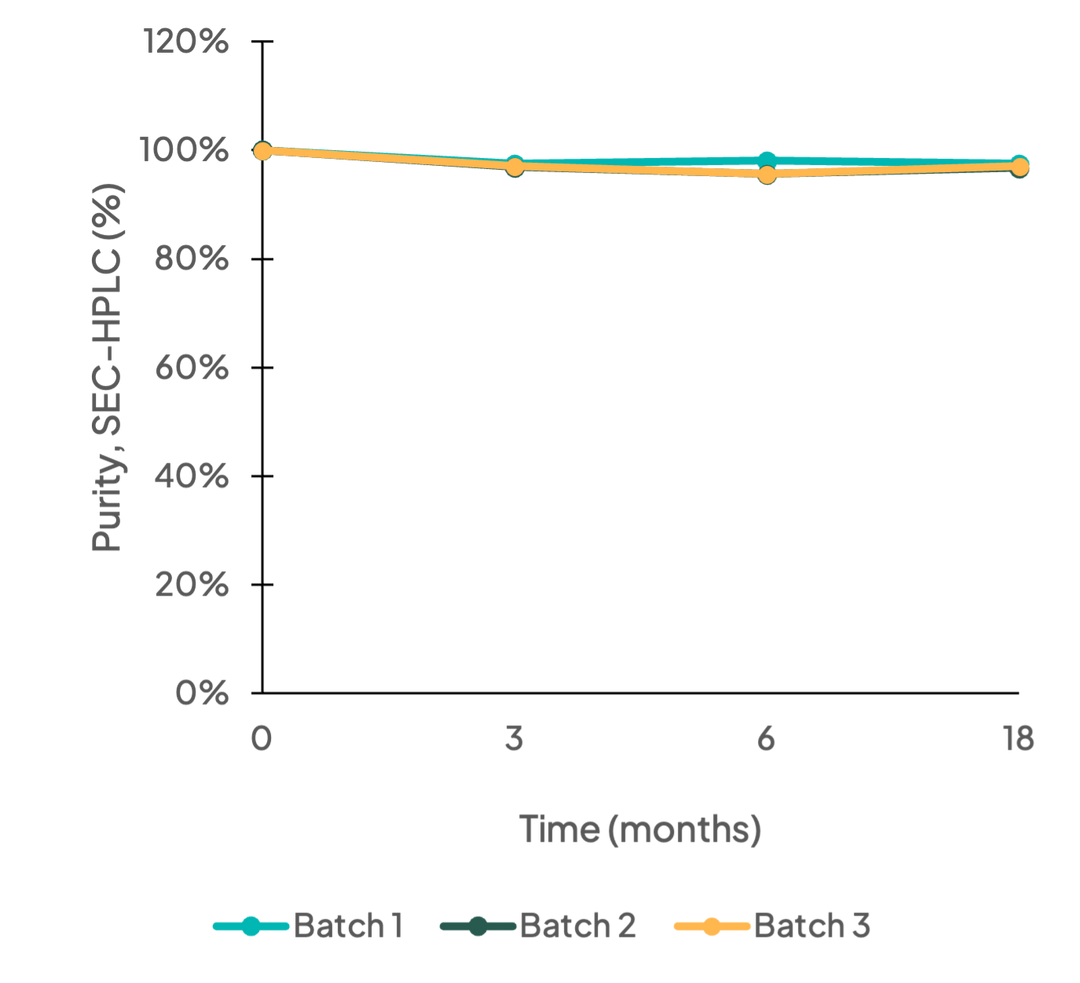
Purity
Long-term stability testing (0-18 months) of three batches of Cas9 nuclease was performed using SEC-HPLC. Results demonstrate the purity of Cas9 nuclease has good long-term stability and batch-to-batch consistency.
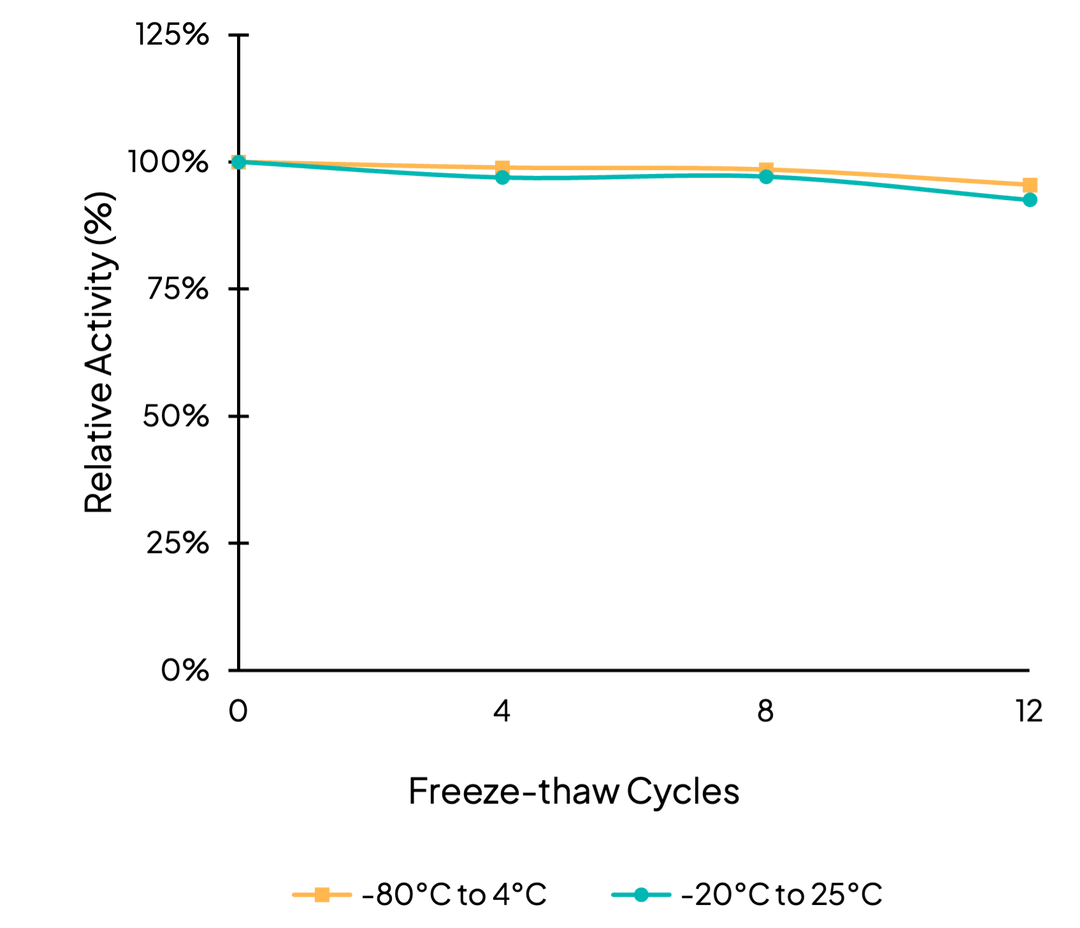
Freeze/Thaw
Accelerated stability testing of MaxNuclease over 12 freeze/thaw cycles between -80C/4C and -20C/25C. Results demonstrate MaxNuclease has consistent activity up to 12 freeze/thaw cycles.
Comprehensive Documentation System
Quality Management System
The Quality Management System (QMS) documentation includes the QMS program overview, guidelines and basic regulations for quality management, and quality system operation.
Specification Program
Our Specification Program includes expansion instructions and concrete documentation of the quality manual, so that the principled and programmatic requirements in the quality manual can be expanded and implemented as necessary.
Criteria Support Documents
Criteria Support Documents includes: Standard Management Procedures (SMP), Quality Standards, Standard Operating Procedures (SOP), Work Instructions (WI), and other supporting documents.
Quality Report
Our Quality Report records the activity status and results of each product, providing evidence that the product meets the quality requirements and the QMS is operating effectively
Customizable GMP Documentation Package
- Datasheet
- CoA
- CoO
- MSDS
- Melamine Statement
- TSE/BSE Statement
- Nitrosamine Statement
- DMF Filing
Our document management system can also provide accurate and traceable Batch Production Records and Batch Inspection Records.
Under the reasonable requirements of customer product declaration and supplier audit, KACTUS can provide bacteria identification and passage stability test reports, inspection method verification, process verification, and regulatory information related to FDA DMF filings, where applicable.
GMP Enzyme Catalog
GMP Enzymes for RNA & Gene Therapy Manufacturing FAQs
GMP-grade enzymes are produced under Good Manufacturing Practice (GMP) standards to ensure product safety, consistency, and regulatory compliance. They are essential in mRNA and gene therapy workflows, where enzyme variability or contamination could compromise product efficacy or patient safety.
GMP-grade products are manufactured under more stringently validated processes with full traceability, extensive documentation, and rigorous quality controls. They meet regulatory expectations for use in clinical trials and commercial therapeutics. Our research-grade enzymes still undergo rigorous QC and are high purity for optimal performance in research experiments, but do not have the same regulatory requirements.
Each lot is tested for identity, purity (via SEC-HPLC), concentration, endotoxins, bioburden, DNase/RNase activity, host cell protein and DNA, heavy metals, and residual solvents. Stability testing ensures performance under both short- and long-term storage conditions.
Manufacturing is conducted in ISO 13485-certified facilities. Several enzymes have supporting Drug Master Files (DMFs) registered with the U.S. FDA, which provides additional regulatory support during IND or BLA submissions.
KACTUS operates 1000L-scale fermentation tanks, high-throughput chromatography systems, and aseptic isolators. Analytical testing utilizes instruments such as HPLC, Q-TOF mass spectrometers, and validated in vitro activity assays.
Standardized expression systems, purification protocols, and manufacturing parameters are used across all batches. Each production run is benchmarked against reference standards to confirm consistent yield, purity, and enzymatic activity.
Stability studies include accelerated degradation testing, long-term storage simulation, freeze-thaw resilience, and transportation stress testing. These studies ensure enzymes retain their functional and structural integrity under real-world conditions.
Each shipment is supported by a full documentation package: Certificate of Analysis (CoA), Certificate of Origin (CoO), MSDS, batch production records, and stability data. Regulatory files such as DMF access letters are available upon request.
Yes. KACTUS supports custom enzyme formats tailored to specific manufacturing needs, including formulation adjustments, concentration specifications, documentation customization, and scale-up for bulk GMP supply.
The 10,000 m² GMP site houses segregated R&D, QC, and production areas. With 1000L fermentation capacity and GMP-grade cleanrooms, the facility enables high-throughput, large-scale enzyme production under stringent environmental and quality controls.








