Surface Plasmon Resonance Assay Services
SPR analysis for protein & small-molecule binding interactions
Surface Plasmon Resonance (SPR) is an optical technology that can detect biomolecular interactions in real time by measuring changes in the refractive index near a sensor surface. This allows for label-free analysis of binding response events between biomolecules, providing precise information on affinity determination, kinetic parameters, and equilibrium dissociation constants (Kd). SPR is widely used in high-throughput screening for drug discovery, fragment screening, and for screening therapeutic antibodies candidates, characterizing molecular interactions, and optimizing binding affinities.
SPR can analyze the binding specificity between biomolecules and perform concentration quantification, affinity determination, kinetic analysis, thermodynamic analyses, and antibody characterization without the need for additional detection reagents, truly reflecting interaction conditions. With its high-throughput, high flexibility, and high sensitivity, SPR has become an important diagnostic method in immunodetection, early drug development, drug-target interactions, and preclinical drug screening. KACTUS offers experienced surface plasmon resonance services, customized for various experimental protocols to meet the unique needs of your research.
Diverse Sample Pairs
Full Assay Development
2-5 Day Turnaround
Regulatory Filing Support

Workscope Discussion
→

Contract Sign-off
→

Method Development
→

Sample Testing
→

Delivery
Includes standard proteins and monoclonal antibodies up to 5 sample pairs
2-3 Days
Includes VLPs, small molecules, or peptides up to 5 sample pairs
3-5 Days
At KACTUS, we are committed to ensuring that our SPR assay development services adhere to the highest standards of regulatory compliance. Our procedures encompass strict supply and management standards for reagents and consumables, meticulous usage records, calibration reports, and regular maintenance for equipment. We prioritize the reliability and integrity of our data through dual backup systems and maintain thorough experimental records in both electronic and paper formats. This rigorous approach guarantees the accuracy and dependability of our SPR analyses, providing you with reliable results for your research and development needs.

Reagents & Consumables:
Strict Material Supply Management

Equipment:
Usage Records, Calibration Reports, & Regular Maintenance

Raw Data Storage:
Dual Backup Systems for Enhanced Reliability

Experimental Records:
Documented in Both Paper and Electronic Copy
SPR is a versatile tool suitable for analyzing the binding interaction between various types of molecules. We offer SPR analysis of the following sample pairs:

Antibody / Protein

Protein / Protein

Protein / Peptide

Protein / Small Molecule
Covalent Immobilization:

Proteins are covalently immobilized on the chip surface through amine coupling. This coupling method involves a reaction with the amino groups of the ligand protein. Generally, amine coupling is used when there are label conflicts with the substances being analyzed.
Non-covalent Immobilization:

Non-covalent methods rely on specific, high-affinity interactions between the protein and a capture molecule on the chip. This approach is excellent for maintaining the correct orientation of the immobilized protein and for recycling the chip surface (except for SA-biotin).
KACTUS SPR Detection Platform is supported by a professional and experienced research team dedicated to developing and optimizing SPR assays. Through strict quality control of our extensive product catalog, our team has accumulated significant technical expertise in SPR analysis, ensuring the quality of our deliverables and an excellent customer experience.
To address the challenge of conducting multiple affinity tests on difficult-to-express transmembrane proteins, KACTUS has specially developed biotinylated virus-like particle (VLP)-displayed antigens to meet the experimental requirements of SPR.
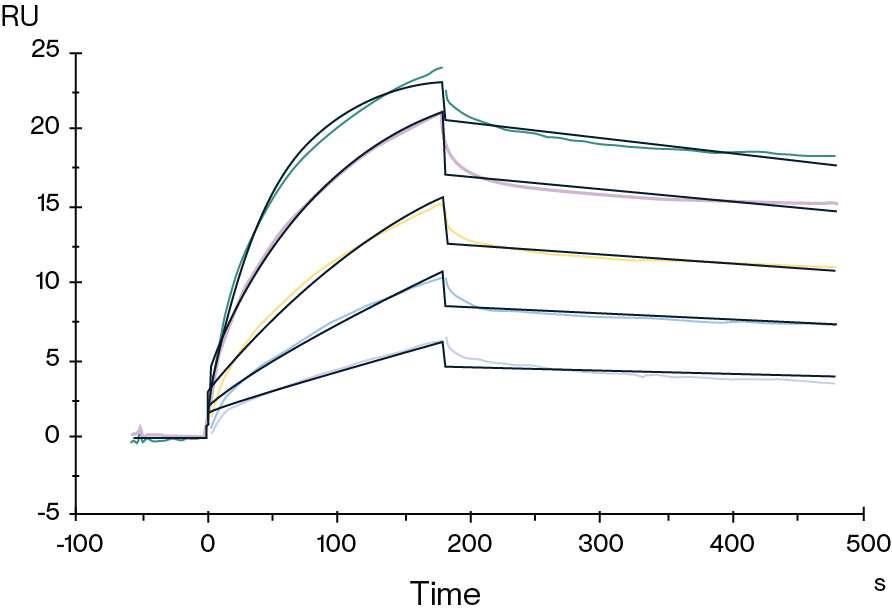
Figure 1. Biotinylated Human Claudin 18.2 VLP captured on CM5 Chip via Streptavidin can bind Anti-Claudin 18.2 Antibody with an affinity constant of 1.28 nM as determined in SPR assay.
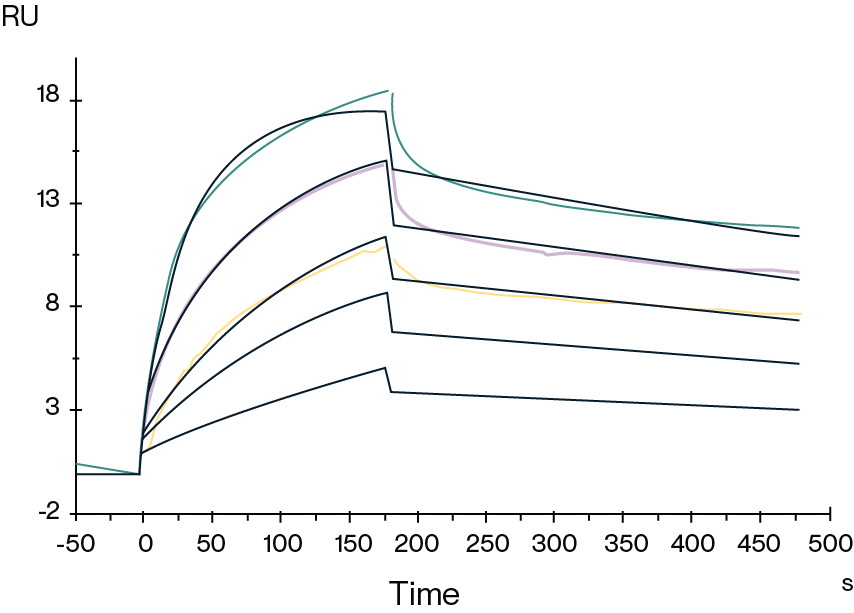
Figure 2. Biotinylated Human GPRC5D VLP captured on SA Chip can bind Anti-GPRC5D antibody, hFc with an affinity constant of 0.30 nM as determined in SPR assay.
Fc receptors are receptor proteins that can bind to the Fc region of antibodies, playing an important role in the screening and efficacy evaluation of antibody drugs. However, this binding interaction is often weak and difficult to effectively assess with conventional detection methods such as ELISA. Therefore, it necessitates the use of methods with higher sensitivity, such as SPR, for detection.
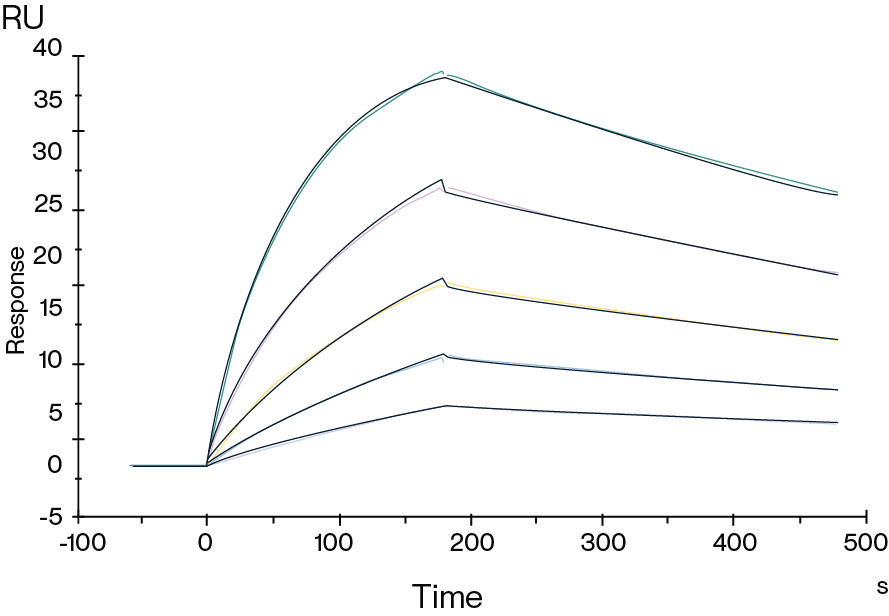
Figure 3. Human Fc gamma RI, His Tag captured on CM5 Chip via anti-his antibody can bind a clinical HER2 antibody with an affinity constant of 1.94 nM as determined in SPR assay.
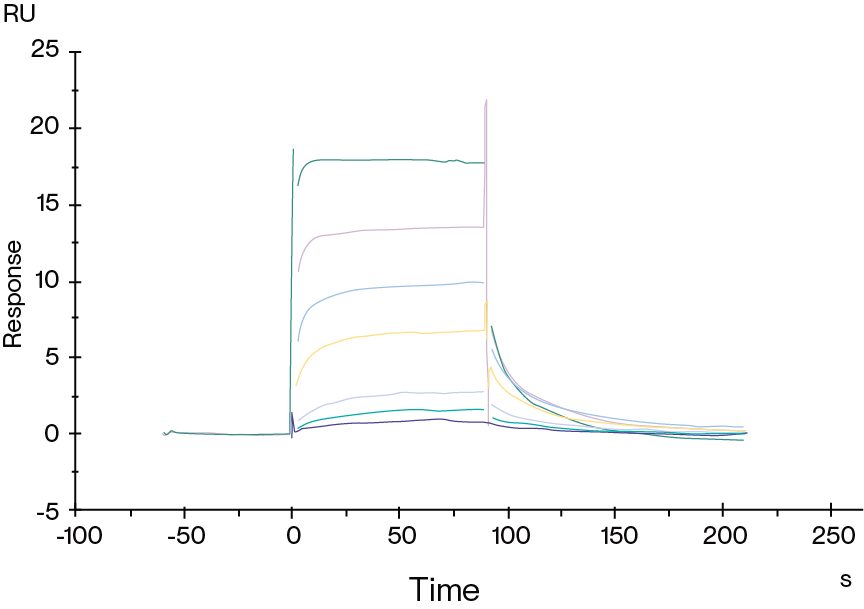
Figure 4. Human FcRn, His Tag captured on CM5 Chip via anti-his antibody can bind Human IgG1 Fc, No Tag with an affinity constant of 0.28 μM as determined in SPR assay.
The detection of the affinity between TCR and MHC peptide complexes is a crucial part of the development of TCR-related drugs, with SPR being a highly effective method for this detection. KACTUS offers SPR detection services to support the development of TCR-related drugs, in addition to our custom expression services for soluble TCRs.
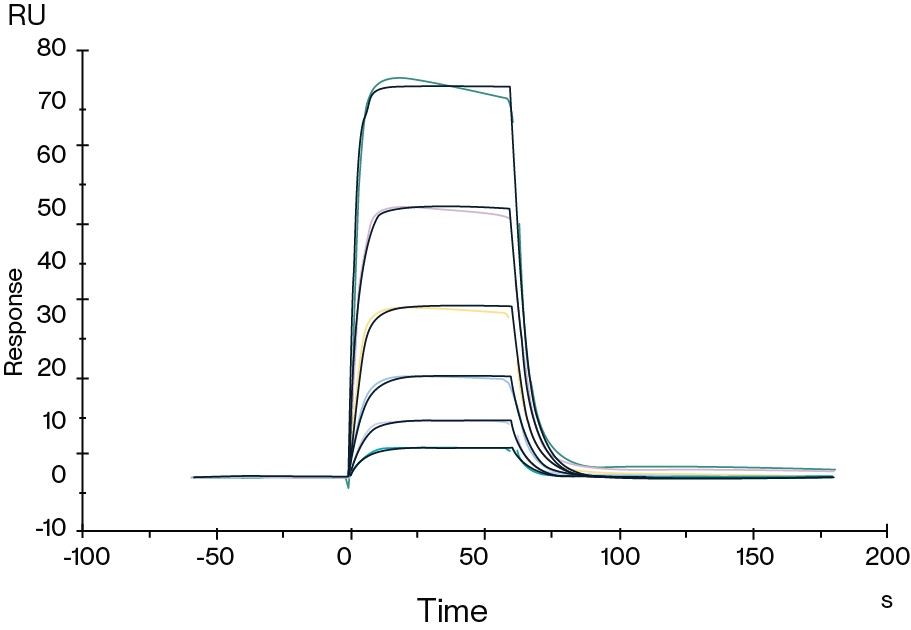
Figure 5. Human HLA-A*02:01&B2M&AFP (FMNKFIYEI) Monomer, His Tag captured on CM5 Chip via Anti-His Antibody can bind HLA-A*02:01&B2M&AFP (FMNKFIYEI) TCR with an affinity constant of 0.923 μM as determined in SPR assay.
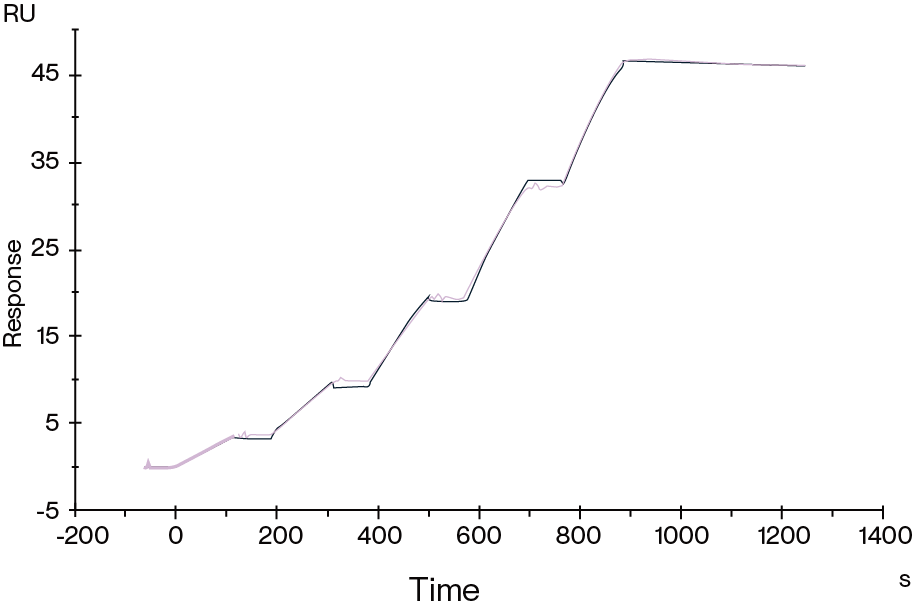
Figure 6. Human HLA-A*02:01&B2M&GP100 (YLEPGPVTA) Tetramer, His Tag immobilized on CM5 Chip can bind gp100 TCR&Anti-CD3 bispecific fusion protein with an affinity constant of 0.196 nM as determined in SPR assay.
SPR is a real-time, label-free biophysical technique that monitors changes in the refractive index at a sensor chip interface as molecular binding events occur. It allows for the precise determination of association (ka) and dissociation (kd) rate constants, from which the equilibrium dissociation constant (Kd) is calculated. It is widely used for characterizing binding kinetics, affinity, and concentration-dependent interactions.
Our SPR services are suitable for a broad range of interaction types, including antibody–antigen, protein–protein, peptide–protein, and small molecule–protein complexes. We also support the analysis of more complex biomolecules such as virus-like particles (VLPs), transmembrane proteins, Fc receptors, and T cell receptor–MHC peptide complexes. These capabilities make our platform highly adaptable to both conventional and challenging binding systems.
Standard assay projects using purified protein or antibody samples are typically completed within two to three business days. For more complex analytes, such as membrane protein nanodiscs, peptides, or multimeric complexes, the workflow may extend to three to five business days. This timeline includes method development, sensor chip preparation, kinetic runs, and data processing. SPR services are performed on a first come first serve basis.
Clients are asked to provide the ligand and analyte identities, their intended roles in the assay (e.g., immobilized vs. injected), the concentration and buffer composition of each sample, and documentation on purity (ideally ≥90%). A minimum volume of 100 µL per interaction is recommended. Following review of your request, our team will guide you in optimizing sample conditions to ensure compatibility with SPR instrumentation and assay parameters. If a tested protein is available in our catalog, we can provide the protein for SPR testing for free.
We utilize both direct and indirect immobilization methods depending on the biochemical properties of the ligand. Direct immobilization involves covalent attachment to the sensor chip via amine coupling. Indirect (capture-based) methods rely on molecules such as streptavidin, Protein A/G, or anti-His antibodies to anchor tagged ligands. These strategies are chosen based on the desired assay orientation, ligand activity, and sample purity requirements.
Our Biacore SPR system is capable of measuring interactions within a broad dynamic range, with affinities from picomolar (pM) to low millimolar (mM), on-rates ranging from 10² to 10⁶ M⁻¹s⁻¹, and off-rates from 10⁻⁶ to 1 s⁻¹. This level of sensitivity enables the characterization of high-affinity therapeutics as well as weak or transient interactions, such as those seen in TCR-pMHC systems or Fc receptor binding.
Upon completion, clients receive detailed kinetic analysis reports, including fitted binding curves, raw sensorgrams, residual plots, and calculated kinetic parameters (ka, kd, Kd). We also provide the raw data files in standard formats (.csv, .txt), along with a Certificate of Analysis if required. Our team is available to provide interpretation support and guidance on result application in your downstream research.
Yes, we routinely handle assays involving weak or transient interactions. Depending on the project, we can adjust surface densities, flow rates, buffer compositions, and regeneration protocols. For interactions approaching mass transport limits or requiring high sensitivity, we implement low-temperature runs and optimized chip chemistries to improve resolution and binding signal fidelity.
While our SPR services are intended for research use, our workflow follows strict SOPs that align with GLP-like standards. We maintain complete documentation, including instrument calibration logs, usage records, dual data backups, and detailed electronic and paper-based experimental records. This makes our assay data appropriate for preclinical reports, early-stage regulatory filings, or investigational documentation.
Absolutely. SPR is routinely applied in lead candidate selection, affinity maturation, epitope binning, and isotype/format comparison. We support screening campaigns for mAbs, bispecifics, and Fc variants, including low-affinity FcγR interactions or pH-dependent binding assessments.