We're upgrading our full protein catalog to ultra-low endotoxin levels.
KACTUS is continually committed to exceptional quality. That's why are taking the initiative to upgrade our entire recombinant protein catalog to endotoxin levels < 0.01 EU/μg or even < 0.001 EU/μg.
We're completing this catalog upgrade no additional cost, and no compromise on performance—just cleaner, more reliable proteins tailored to your drug development needs. These ultra-low endotoxin proteins are ideal for:
To request an ultra-low endotoxin protein, please contact your sales representative or reach out to us at sales@kactusbio.us.
Advantages of KACTUS Low Endotoxin Proteins
→ Ultra-Low Endotoxin: Endotoxin levels <0.01 or <0.001 EU/μg—surpassing industry standards and minimizing the risk of immune interference.
→ High Purity: >90% purity confirmed by Tris-Bis PAGE for consistent performance.
→ Stringent Quality Control: Every lot is tested for purity and endotoxin content to ensure batch-to-batch consistency.
→ Immunization-Ready: Suitable for animal immunogenicity studies and injections, reducing the risk of endotoxin-driven inflammatory responses.
→ Assay-Compatible: Optimized for ELISA and flow cytometry—minimizing background signal by reducing endotoxin interference with antigen–antibody binding.
Why choose KACTUS for low endotoxin proteins?
Custom Low Endotoxin Protein Expression Services
KACTUS offers custom recombinant protein expression services tailored to meet stringent endotoxin requirements for preclinical and immunotherapy research. Our optimized expression systems—paired with purification workflows designed to minimize endotoxin contamination—enable the production of high-purity proteins with endotoxin levels as low as <0.001 EU/μg. Whether for animal immunization, functional assays, or preclinical studies, we deliver high-quality proteins expressed in optimized systems and rigorously purified to meet strict endotoxin specifications—ensuring both biological activity and compatibility with sensitive applications.
To request custom ultra-low endotoxin proteins, please contact your sales representative or reach out to us at sales@kactusbio.us.
Frequently Asked Questions
How does KACTUS test endotoxin levels?
To ensure the reliability of our ultra-low endotoxin proteins, we use the Limulus Amebocyte Lysate (LAL) assay, the industry-standard method for endotoxin detection. This highly sensitive test allows us to accurately quantify endotoxin levels down to 0.001 EU/μg, verifying that our products meet the stringent thresholds required for immunization, in vitro screening, and preclinical applications.
Each batch is tested under controlled conditions, and results are documented as part of our quality control profiles.
What are endotoxins?
Endotoxins are lipopolysaccharides (LPS) found in the cell walls of Gram-negative bacteria. They consist of three components: lipid A (the toxic center), a core polysaccharide, and an O-antigen. Endotoxins may be introduced during recombinant protein production, particularly when using prokaryotic expression systems such as E. coli. In eukaryotic expression systems, endotoxins are typically introduced from prokaryotic-derived plasmids.
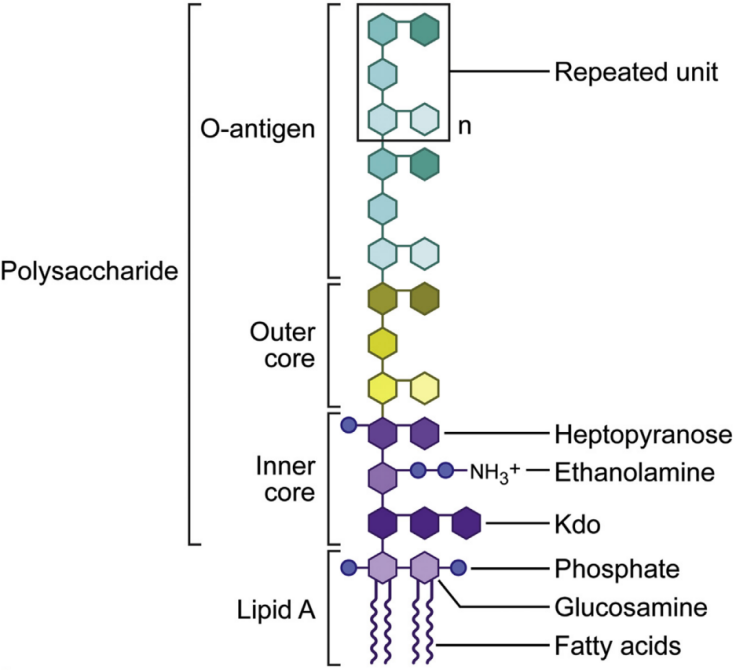
Endotoxin molecular structure [1].
Importance of endotoxin control in antibody drug discovery
High endotoxin levels in recombinant proteins can compromise multiple stages of the antibody discovery process. During animal immunization, endotoxins may induce inflammatory responses, dysregulate the immune system, and result in low antibody titers or increased production of non-specific antibodies. During in vitro screening stages such as ELISA or flow cytometry, endotoxins can interfere with antigen–antibody interactions, leading to high background noise and reduced screening accuracy. Therefore, stringent control of endotoxin levels is essential when producing recombinant proteins for drug research and development.
Endotoxins are heat-stable and resistant to conventional removal methods such as heat treatment, making them particularly challenging to eliminate. Therefore, effective control of endotoxin requires interventions at every step—from expression system design to downstream purification. At KACTUS, we are committed to elevating protein quality by optimizing expression conditions and refining purification workflows. This allows us to consistently achieve ultra-low endotoxin levels, reducing the risk of immune interference and better fulfilling the stringent needs of animal immunization and preclinical drug development.