Breaking News: KACTUS GMP-Grade Cas9 Powers Bioheng’s Breakthrough IND Clearance for Next-Gen UCAR-T Therapy
By Mallory Griffin
KACTUS is thrilled to announce that our GMP-Grade Cas9 enzyme — part of our portfolio of proteins and enzymes for cell therapy — is playing a pivotal role in the development of Bioheng Therapeutics' revolutionary UCAR-T cell therapy! In a major milestone, Bioheng's cutting-edge UCART cell product received Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration (FDA) on March 1, 2025.
This FDA approval marks a significant breakthrough for Bioheng, propelling their lead pipeline into the global clinical stage and highlighting the critical role of high-quality gene editing enzymes in advancing next-generation cell and gene therapies. It also underscores KACTUS’ reputation as a top-tier global supplier of high-quality, GMP-Grade Cas9 Enzyme, essential for advancing cell and gene therapy (CGT).
Learn more about KACTUS Cas9 enzyme →
About CTD-402
CTD402 is a universal chimeric antigen receptor T (UCAR-T) cell therapy targeting CD47, intended for the treatment of pediatric and adult patients with relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoma (R/R T-ALL/LBL). This cell product is derived from healthy donors and genetically modified to prevent graft-versus-host disease (GvHD), and host-versus-graft rejection (HvG) while enhancing anti-tumor activity. CTD402 can be produced in one large single batch for multiple doses and patients, providing an "off-the-shelf" solution for patients in need of CAR-T cell therapy.
Currently, Bioheng is planning to proceed to a Phase Ib/II clinical trial with a simplified dose exploration design.
Supplying GMP-Grade Cas9 Enzyme for Clinical Manufacturing
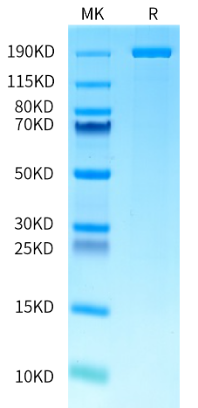
KACTUS is the partner of Bioheng as the exclusive supplier of GMP-Grade, high-activity Cas9 enzyme and provides IND application support throughout this process. Known for its high activity and batch-to-batch consistency, KACTUS GMP Grade Cas9 enzyme has been successfully applied in multiple clinical manufacturing processes since product launch. We are committed to providing high-standard gene editing tools for cell and gene therapy (CGT) companies. With nearly 10,000 square meters of GMP manufacturing facilities and a certified quality management system, we have established multiple off-the-shelf gene editing enzymes:
GMP-Grade Cas9 Enzyme – Trusted by nearly 100 pharmaceutical companies worldwide, supporting the full CGT development cycle from early research to clinical application.
Catalog No. GMP-CAS-EE109
GMP-Grade Cytosine Base Editor AccuBase® – Used in FDA-approved IND applications for relevant clinical pipelines.
Catalog No. GMP-KD-0001
Cas9 Enzyme ELISA Kit - Universal Cas9 enzyme detection kit for quantifying Cas9 residues.
Catalog No. CAS-MM00B
GMP-Grade Cas9 Enzyme Performance Data
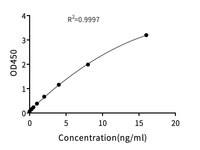
We've conducted extensive performance and analytical testing on our Cas9 enzyme to ensure high editing activity and consistent quality. Our GMP-grade Cas9 enzyme demonstrates strong editing activity across various cell types, comparable to industry standards. KACTUS has demonstrated successful gene knockouts in multiple cell lines, including 293T, Jurkat, and primary T cells, with editing efficacies greater than 85%. We have also conducted stability testing on the Cas9 enzyme to ensure its reliability for use in clinical applications.
Click here to learn more about our Cas9 enzyme →
Gene Knockout Efficiency

Figure 1. Gene knockout of TRAC in primary T cells with different batches of KACTUS GMP-grade Cas9 enzyme. Results show greater than 90% editing efficacy across all three batches, comparable to a leading supplier. Briefly, 75 pmol of Cas9 enzyme was electroporated along with 225 pmol of sgRNA into various cells using the Lonza 4D Nucleofector system. The cells were cultured for three days followed by TIDE analysis.

Figure 2. Gene knockout efficiency of BCL11A in hematopoietic stem cells (HSCs) for the treatment of β-thalassemia using Cas9 nucleases from KACTUS and two leading suppliers. Results demonstrate that KACTUS Cas9 nuclease achieves gene knockout efficiency comparable to that of the leading suppliers, validating its effectiveness for therapeutic applications.
Stability Testing

Figure 3. Consistent activity was observed across three manufacturing batches of KACTUS GMP-grade Cas9. Three batches of KACTUS GMP-grade Cas9 were stored at -20°C for long-term stability testing. In vitro cleavage activity was analyzed at 3, 6, 9, 12, and 18 months, respectively. Results show our GMP-grade Cas9 has consistent activity across time and different manufacturing batches.
Request a test sample of KACTUS Cas9 enzyme →
Looking forward, KACTUS will continue to maintain the current cGMP production process and strict quality control, ensuring agile responses and stable supply chains to empower our partners in accelerating cell and gene therapy (CGT) development. Together, we strive to drive innovation in the global CGT industry.
About Bioheng Therapeutics
Bioheng Therapeutics is a clinical-stage company specializing in the development of allogeneic, "off-the-shelf" universal CAR-T therapies. Founded in 2017, the company is dedicated to advancing globally leading allogeneic cell therapy platforms and products to address significant unmet clinical needs in hematologic malignancies and autoimmune diseases.
About KACTUS
KACTUS is an innovation-driven company specializing in recombinant proteins and enzyme raw materials, dedicated to supporting biopharmaceutical development and manufacturing. With in-house GMP manufacturing facilities and a certified quality system, KACTUS has established a strong portfolio of gene editing enzymes for the cell and gene therapy field, including: