New AAV Purification Strategy, Kactus AAV ELISA Kits Offer Reliable Support
By Natasha Slepak
A recent study published in the Journal of Chromatography A introduced a novel anion-exchange chromatography (AEX) method using a choline chloride (Choline-Cl) salt gradient for purifying adeno-associated virus (AAV). Developed by Tosoh Bioscience, this approach significantly increases the proportion of full AAV capsids to nearly 90%, offering a promising new path for downstream AAV processing.
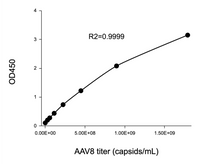
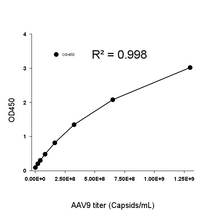
In this study, the Kactus AAV8 Titration ELISA Kit was used to accurately quantify intact AAV8 capsid. Combined with AEX-FL analysis for empty vs full capsid ratio (Table 1) and cross-validated via SEC-UV-MALS (Table 2 in the paper), the results demonstrated that the new purification method effectively enriched full AAV capsids up to nearly 90%, outperforming traditional NaCl gradient elution.

Table 1. Full capsid recovery calculated by ELISA and AEX-FL assay

Analytical AEX-FL profiles showing enrichment of full AAV capsids with the choline-based method. (A) Load sample before polishing shows distinct peaks for empty and full AAV capsids. (B) After purification on a 1 mL AEX column with 200 mM choline-Cl, the eluate is strongly enriched for full capsids, with the empty capsid peak nearly eliminated. These data illustrate the effectiveness of the choline-based anion-exchange chromatography method in achieving high-purity full AAV preparations.
Why Separate Empty and Full Capsids?
During AAV production a significant number of empty capsids, which lack therapeutic genes, are generated. These not only fail to deliver treatment but may also trigger immune responses, compromising drug safety and efficacy. Efficient separation of empty and full AAV capsids remains a critical challenge in commercial gene therapy manufacturing.
Highlights of the Kactus AAV Titration ELISA Kit
The combination of ELISA and AEX-FL confirmed both high full-capsid ratio and excellent repeatability and scalability of the method, supporting clinical-grade AAV production.
-
Accurate quantification: Delivers reliable data for viral titer and recovery rate before/after affinity purification and during AEX polishing.
-
High sensitivity & specificity: Performs robustly even in complex sample matrices, including crude pre-purification samples.
-
Supports process development & QC: Provides key data for optimizing downstream purification and final product quality assessment.
Why Choose Kactus AAV Titration ELISA Kits?
-
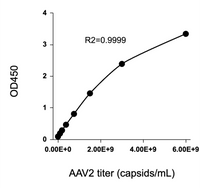
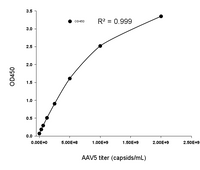
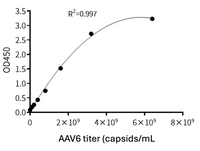
Available for multiple natural serotypes: AAV2, AAV5, AAV6, AAV8, AAV9
-
High sensitivity and broad linear range—ideal for various development stages
-
Strict QC ensures high batch-to-batch consistency
-
Full technical support and custom AAV ELISA development services
From R&D to production, Kactus helps you accurately quantify AAV capsids and accelerate research progress.
Contact us to learn more or request a free sample!
Reference
McAnany Y, Bonakdar L, Bouskila A, Li T, Baun D, Kurth S, Makino-Manabe Y, Tanaka T, Müller E, Dabre R, Kervinen J. Preparative choline-based anion-exchange chromatography for enrichment of full adeno-associated virus capsids. J Chromatogr A. 2025 Oct 11;1760:466319. doi: 10.1016/j.chroma.2025.466319. Epub 2025 Aug 25. PMID: 40886676.
Related Articles
AAV Capsid Optimization and Precision Quantification: Customized AAV ELISA Detection Solutions
Ensuring Safe and Effective Gene Therapy with Precise AAV Capsid Quantification