Meet SAMS™
Structure Aided Multiplex Screening
SAMS™, our proprietary high-throughput protein engineering and expression screening platform, is designed to overcome the challenges of expressing complex proteins. It starts with sequence and structural analysis of the protein before determining optimal expression conditions to maintain yield, purity, and integrity of each protein. SAMS™ ensures reliable production of high-quality proteins, even for the most difficult-to-express targets.
Why SAMS™?
Recombinant protein expression is becoming increasingly specialized.
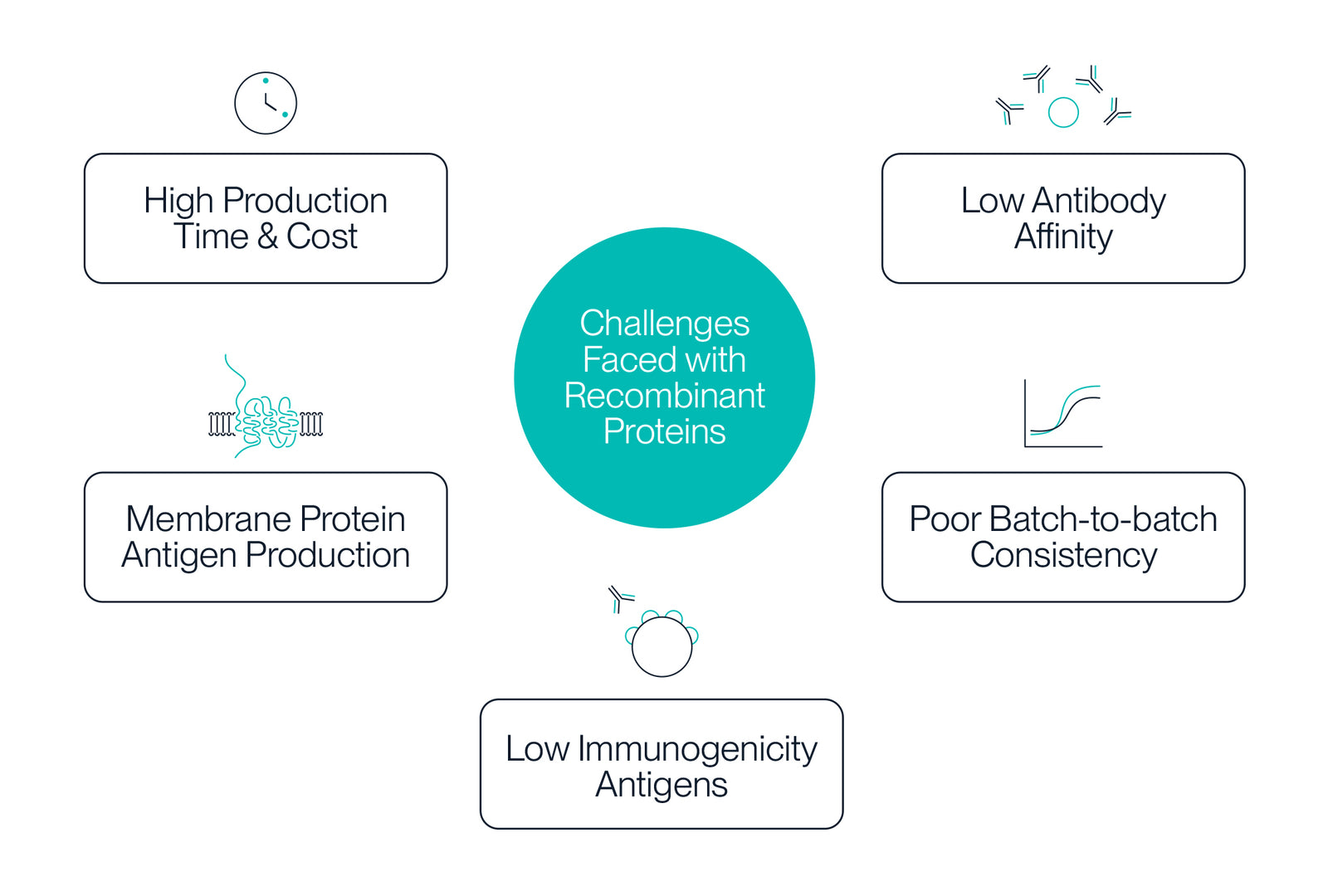
Various bacterial and mammalian production systems are available depending on both the protein and the application. With increasing complexity of therapeutics, protein expression and integrity is of the utmost importance. As researchers continue to target more complex proteins, such as multi-transmembrane proteins, the purification and characterization of recombinant proteins becomes increasingly specialized, with more sophisticated techniques to isolate and analyze proteins with specific properties. Still, researchers face common challenges when using recombinant proteins including:
- Membrane protein antigen production
- Recombinant antigen immunogenicity
- Antibody affinity
- Batch-to-batch consistency
- Production time & production cost
Advantages of SAMS™

Convenience
- Affordable rates & consistent communication for custom projects
- Optimized expression allows for high purity & high yield
- Timely delivery of specialized or difficult-to-express target
Quality
- Bioactivity & purity testing
- Low endotoxin & excellent stability
- High degree of batch-to-batch consistency

Protein Engineering
- Individual structure-based antigen design
- Enhanced immunogenicity & bioactivity
- Mimics native antigen conformation
How SAMS™ works: Bioinformatics, 3D Modeling, & Expression Testing
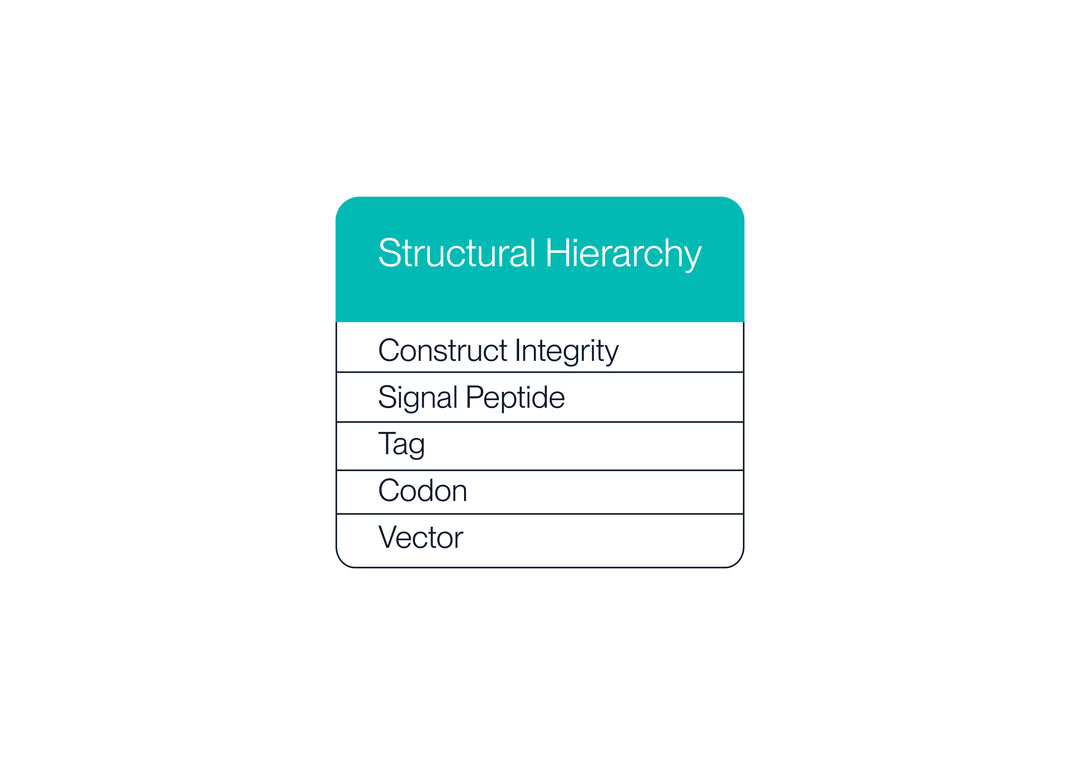
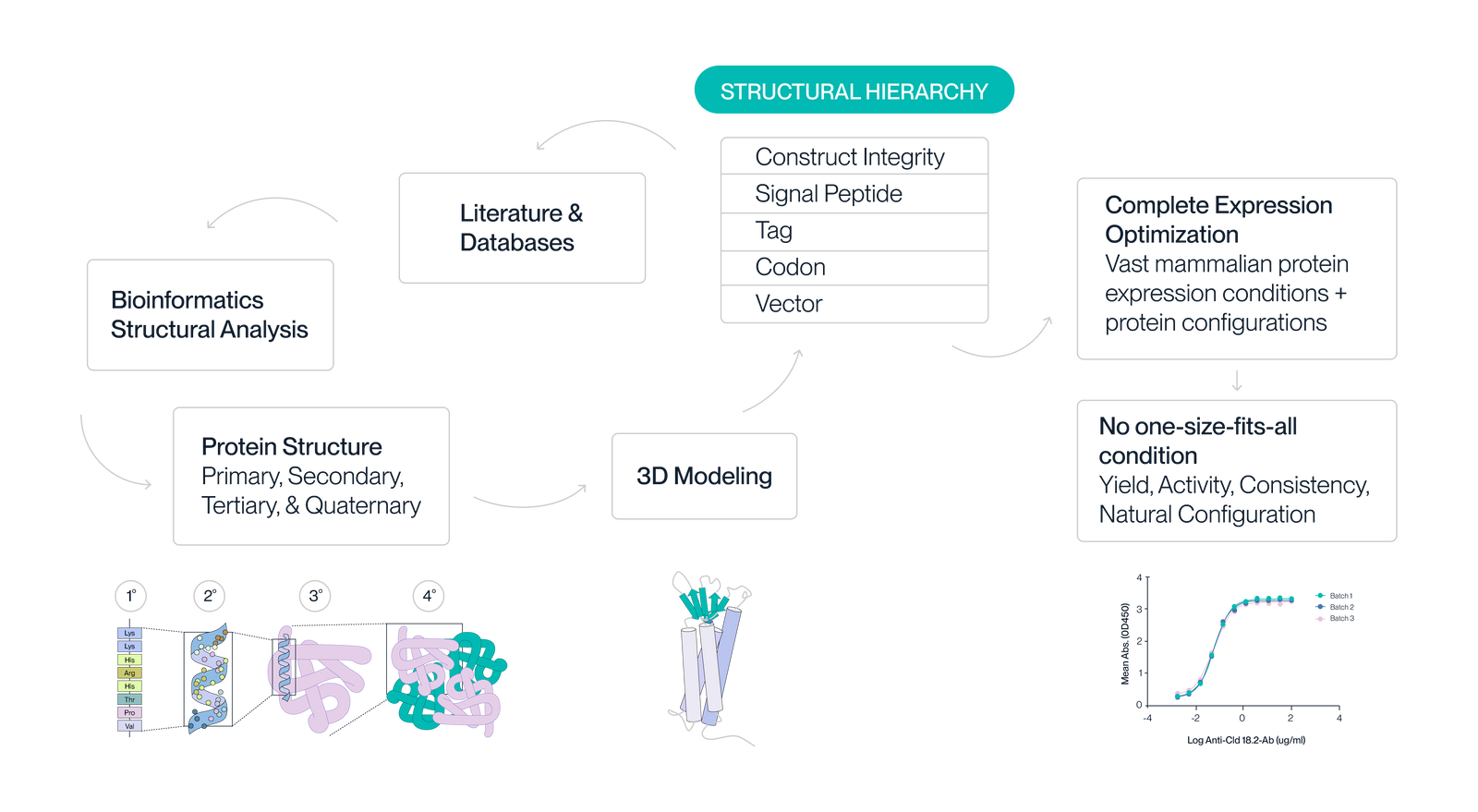
1. Structural Hierarchy
The structural hierarchy is the overarching list of variables that will affect protein structure and function.
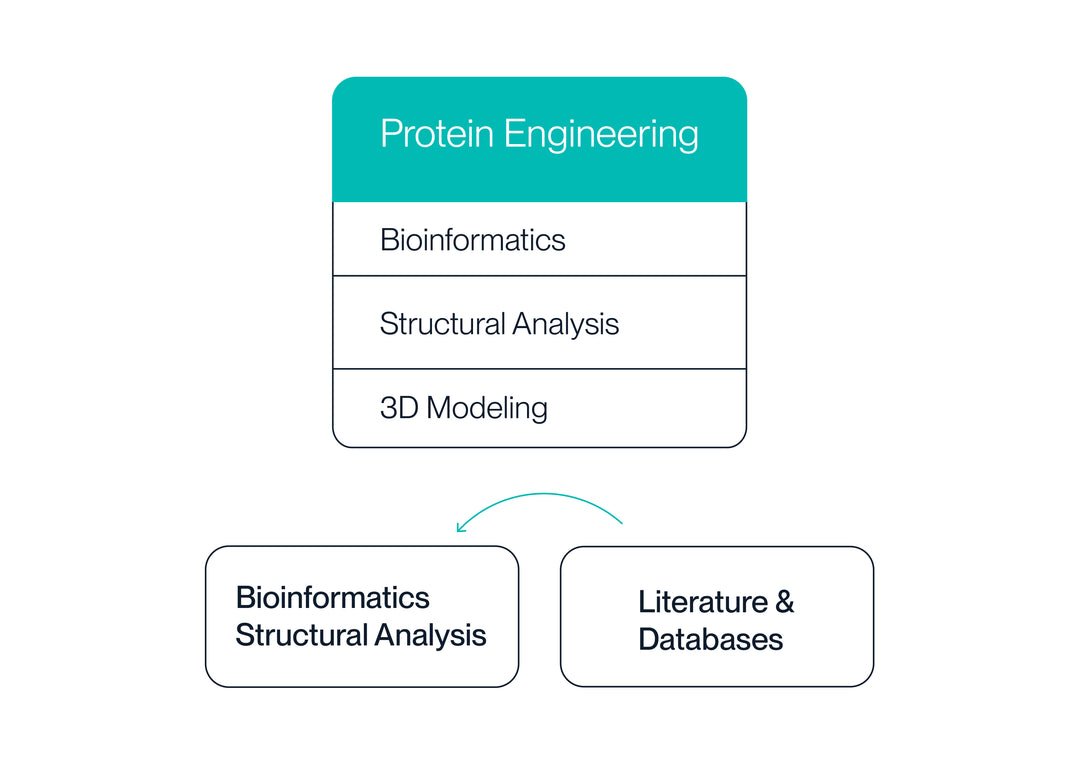
2. Protein Design
Unmet needs for commercial proteins are identified via literature and database searches. We then use bioinformatics and structural analysis to design a variety of protein sequences/structures based on variables from the structural hierarchy.
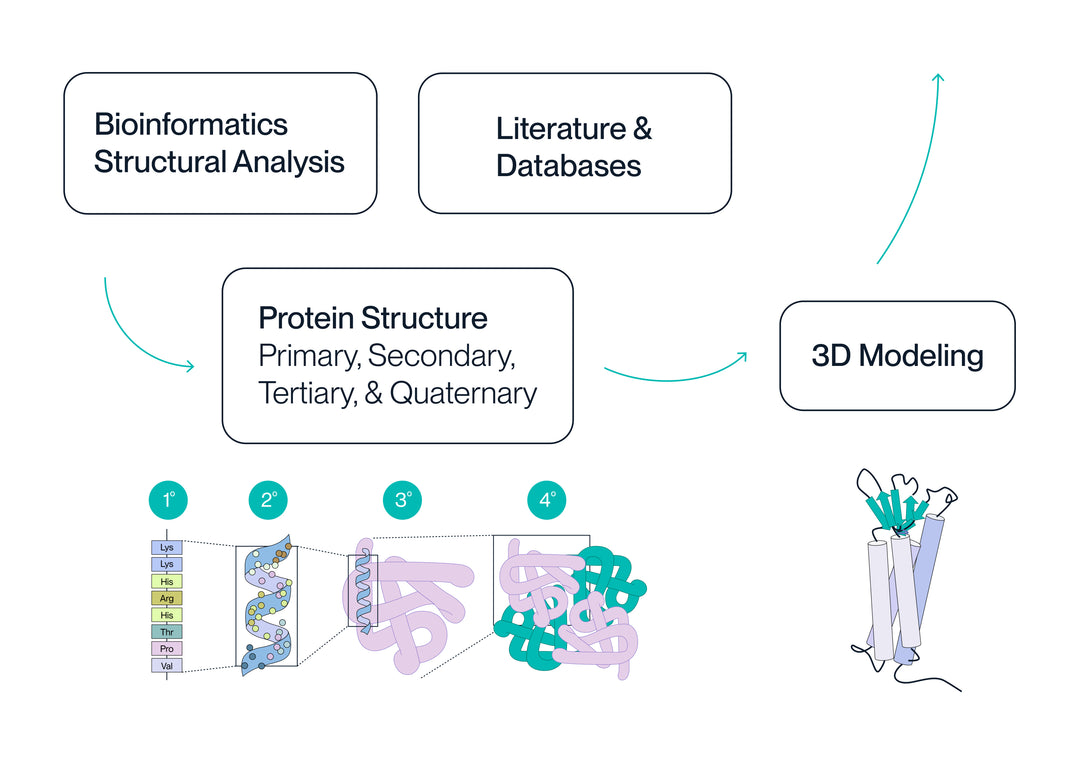
3. Structure Optimization
Integrity, signal peptide, codons, etc. are analyzed using structural informatics and 3D modeling to determine how they will influence all four levels of protein structure.
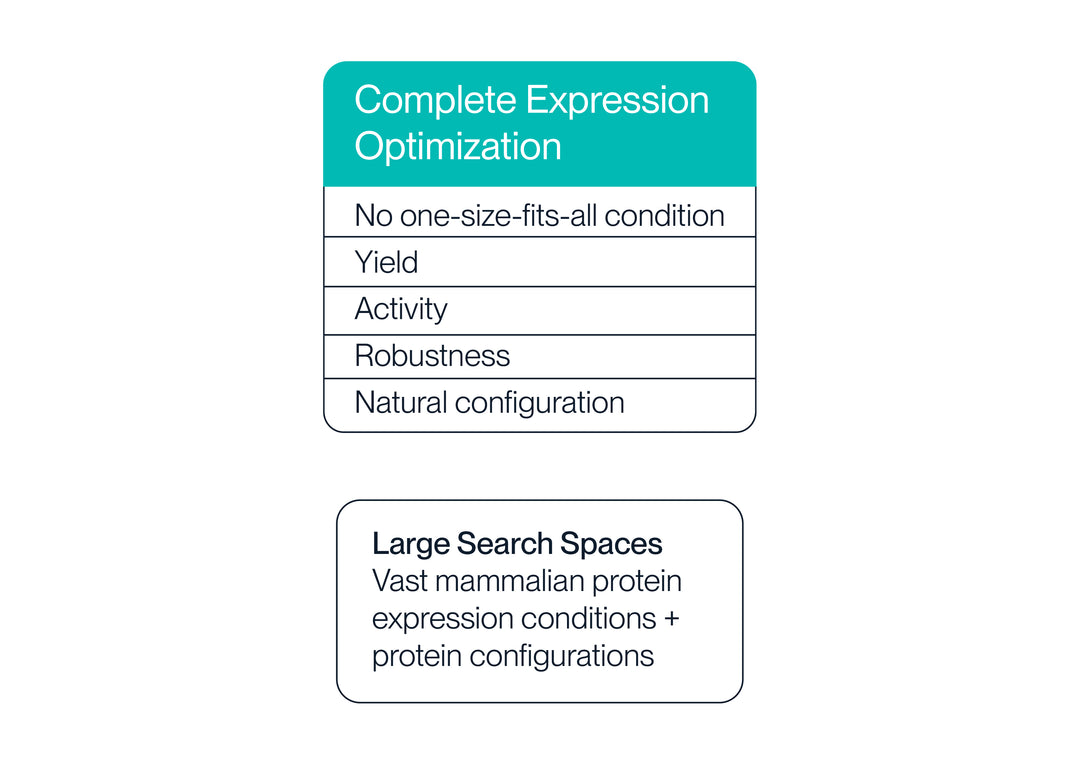
4. Expression Optimization
The optimal protein configuration is identified from large search spaces of protein configurations and expression conditions. We prioritize natural protein configuration, yield, and bioactivity.
5. Performance Validation
Protein designs are tested for bioactivity in multiple cell types/settings. Because we ensure protein function in multiple settings, you can be confident that the protein you purchase will work for your application.
Success with SAMS™
First Full-Length Claudin 18.2
Demonstrating the strength of our SAMS™ technology platform, KACTUS was the first company to produce full-length Claudin 18.2, a popular drug discovery target featuring four transmembrane domains.
Today, our products are used worldwide for diverse applications, including basic research, drug discovery, diagnostic study, antibody production, quality control testing, and more.
Biotinylated Human Claudin 18.2 VLP captured on CM5 Chip via Streptavidin can bind Anti-Claudin18.2 Antibody with an affinity constant of 1.28 nM as determined in SPR assay (Biacore T200).
Let SAMS™ help you
We offer custom recombinant protein production services, including MHC Complexes, VLP-Displayed Antigens, CRISPR Enzymes, and other difficult-to-express proteins.
Structure Aided Multiplex Screening (SAMS™) FAQs
SAMS™ is a proprietary platform developed by KACTUS for engineering and optimizing recombinant proteins. It enhances expression yield, structural integrity, and bioactivity—especially for proteins that are typically challenging to express.
SAMS™ is effective for a broad spectrum of proteins, including membrane-bound targets, multi-pass transmembrane proteins, low-immunogenicity antigens, and conformationally complex proteins used in research areas such as oncology and immunotherapy and diagnostic research.
SAMS™ combines bioinformatics, 3D modeling, and large-scale expression screening to identify optimal coding sequences and expression conditions for each protein target.
The process includes structural hierarchy analysis, protein design, structure optimization, expression testing, and performance validation across multiple biological systems.
Proteins are engineered to mimic native conformations, improving structural integrity and immune response, which is especially important for use in vaccine and antibody discovery workflows.
3D structural modeling is used to analyze codons, signal peptides, and folding domains to inform rational protein design and predict expression success before lab validation.
Yes. SAMS™ is used to develop custom constructs including peptide-MHC complexes, VLP-displayed proteins, CRISPR enzymes, and clinical-grade antigens. Projects can be tailored for research or GMP-level production.
Proteins are tested for bioactivity in multiple biological systems to confirm stability, functional binding, and relevance to the intended application.
SAMS™ delivers higher expression yields, reduced endotoxin levels, and improved lot-to-lot consistency. It also accelerates development timelines for proteins that are difficult to express or prone to misfolding.
The platform has enabled production of previously intractable targets, such as full-length Claudin 18.2, a multi-transmembrane protein with high structural complexity, produced in functional, bioactive form.






