Applications |
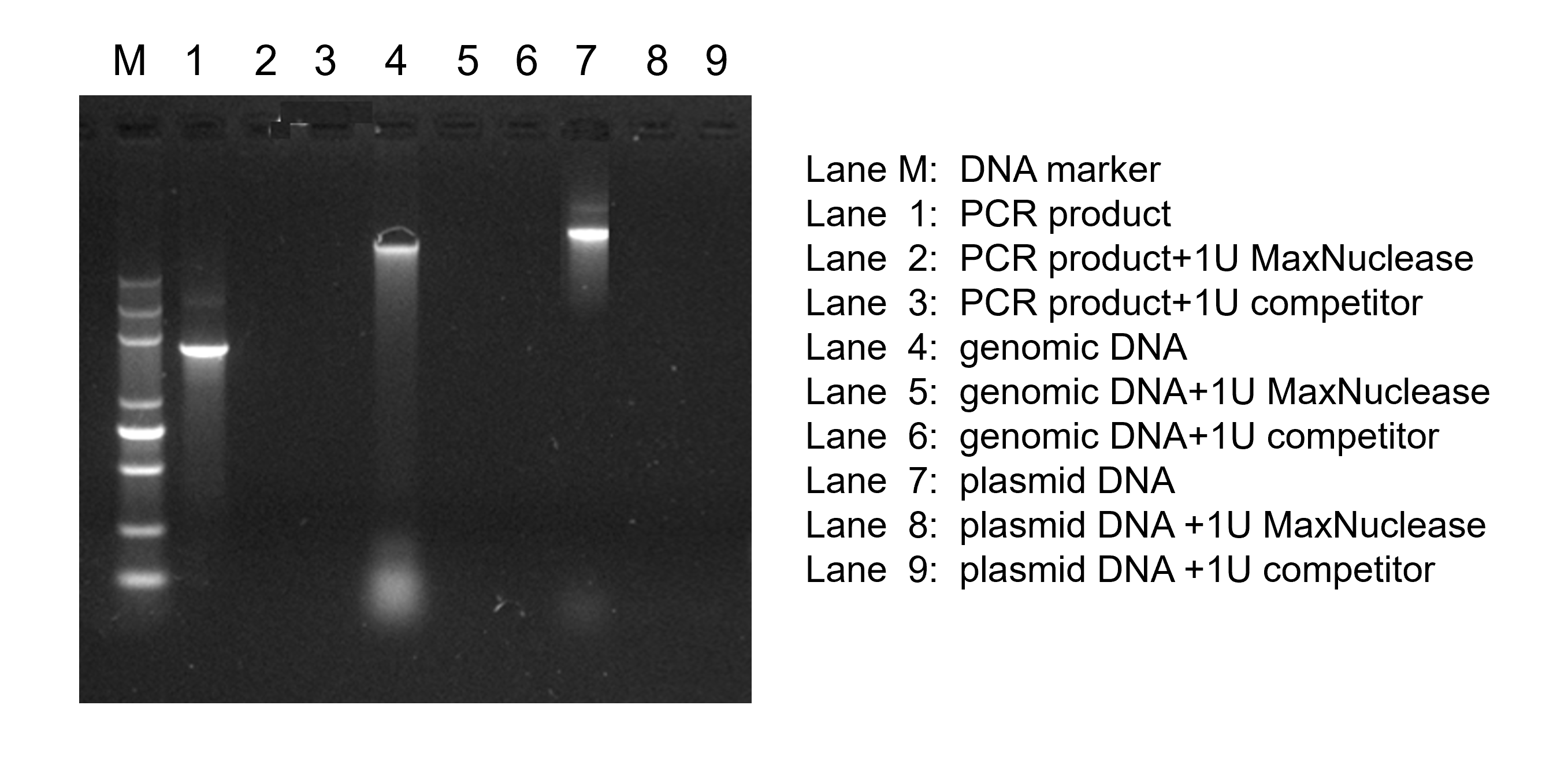
Removing DNA/RNA from other biologicals.
Reducing viscosity caused by nucleic acids, Purification of viral vaccines, viral vectors for vaccine.
Preparing samples in western blot analysis, two-dimensional gel electrophoresis, ELISA and chromatography.
Preventing cell clumping. |
Concentration |
≥ 250U/µL |
Unit Definition |
One unit is defined as the amount of enzyme required to produce a change in absorbance at 260nm of 1.0 in the time of 30 minutes, under optimum conditions with excess substrate. |
Source |
Expressed in E.coli that carries the nuclease gene from Serratia Marcescens. |
Biotinylated |
no |
Molecular Weight |
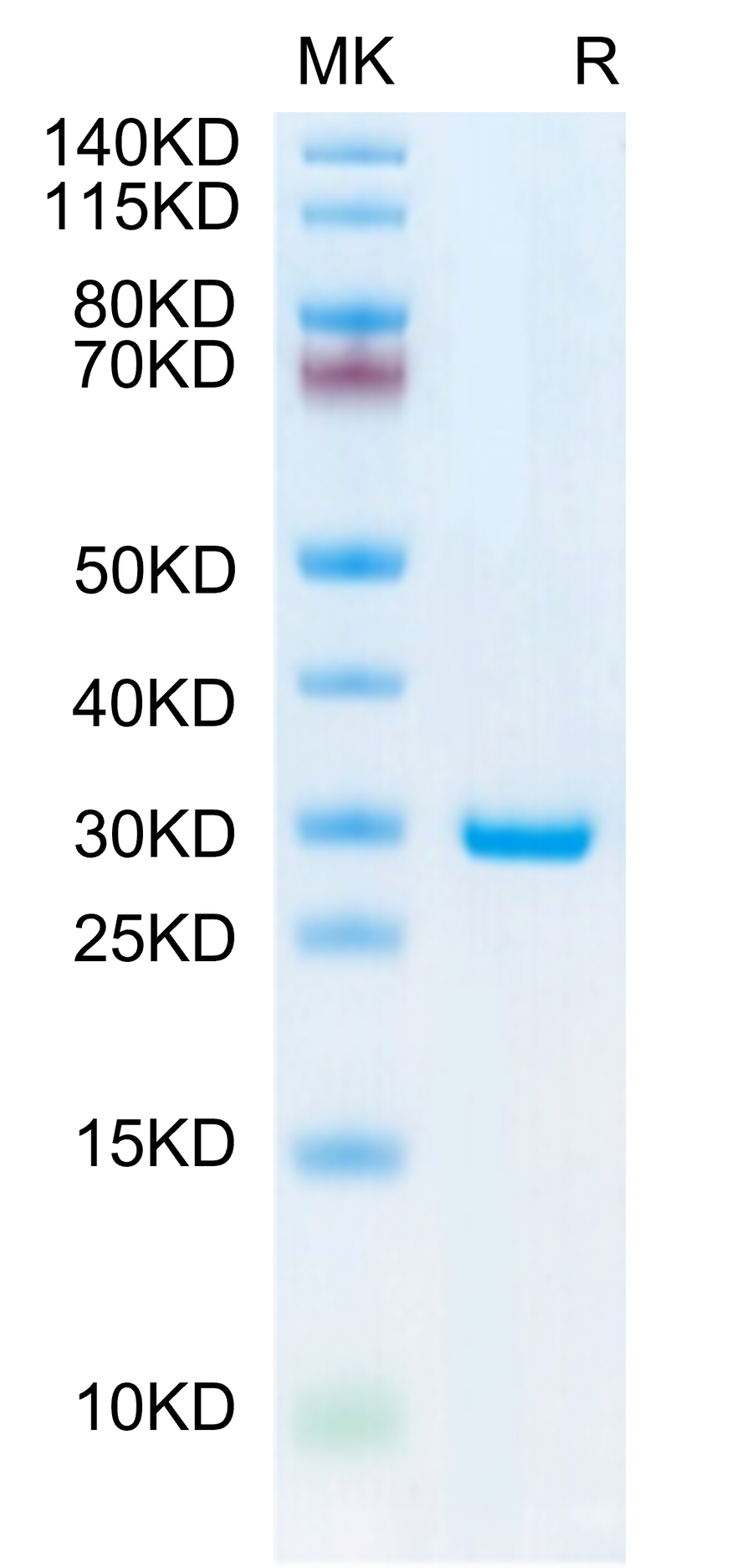
27.8kDa |
Quality Standards |
Activity (Dissolve herring sperm DNA): ≥ 250 U/µL
Purity (Bis-Tris): ≥ 95%
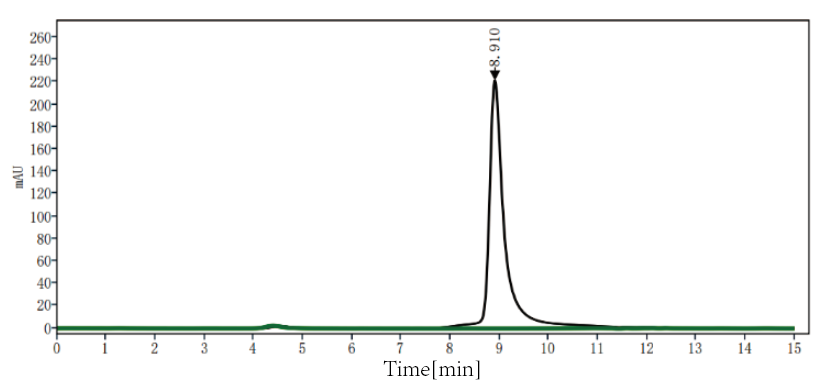
Purity (SEC-HPLC): ≥ 99%
Residual Protease: Negative
Endotoxin: ≤ 0.01 EU/kU
Residual Host Protein: ≤ 10 ppm
Sterility: Negative
Residual Heavy Metal: ≤ 10 ppm
Mycoplasma: Negative
|