AccuBase™ Base Editor Enzyme: Globally In Stock Now
By Yujiao Zhang
Overview
AccuBase™ is a new generation base editing tool independently developed by Base Therapeutics with exclusive global IP rights. AccuBase™ enzyme is characterized by high editing activity and extremely low off-target rates. By leveraging its advanced enzyme design platform, KACTUS has successfully manufactured GMP-grade AccuBase™ enzyme and obtained authorization from Base Therapeutics for global manufacturing and sales as of November 2023. KACTUS and Base Therapeutics are providing bio-pharmaceutical customers with on-hand GMP-grade base editor tools, jointly promoting the development of the cell and gene therapy field.
State of Gene Editing Technology
The future has arrived; the novel CRISPR technology has been implemented and approved as a drug in just a decade. On November 16, 2023, the UK Medicines and Healthcare products Regulatory Agency (MHRA) officially announced the approval of the CRISPR gene editing therapy Casgevy, jointly developed by Vertex Pharmaceuticals and CRISPR Therapeutics, for the treatment of Sickle Cell Disease (SCD) and Transfusion-Dependent β-Thalassemia (TDT) patients. This is undoubtedly a pioneering milestone event, significantly boosting the gene therapy industry and pushing the medical field into a new era.
Multinational companies have also heavily invested in their early layouts for gene editing tools. Recently, Eli Lilly invested $50 million in Beam Therapeutics and acquired the option to purchase several base editing treatment projects of its partner Verve Therapeutics for a total amount of up to $600 million, thus strengthening its pipeline in cardiovascular diseases. One important reason for their significant investment is to acquire the base editor technology from Beam Therapeutics, known as "CRISPR 2.0", which theoretically does not need to damage the DNA double-strand structure and can perform precise gene editing for safer drug development.
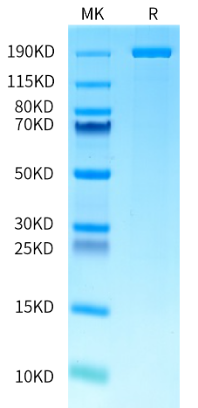
Expression & Manufacturing of AccuBase™
Many scientists and biotech companies are playing significant roles in developing new base editors and making breakthroughs and layouts in underlying patent technology via independent research and development. Among all of them, AccuBase™ is a new generation of base editing enzymes independently developed by Base Therapeutics with exclusive global IP. These types of enzymes are often difficult to express due to their fusion protein nature, or low and unstable yields, making them extremely challenging to be used as an off-the-shelf, ready-to-use enzyme, which is a well-recognized challenge in the industry. Impressively, KACTUS has overcome this challenge and successfully produced GMP-grade AccuBase™ base editor with high yield and activity. KACTUS and Base Therapeutics are now providing convenient, high-quality enzyme tools for a wide range of gene editing applications.
Principles and Advantages of Base Editors
DNA base editing technology is a new genome editing technology developed based on the CRISPR/Cas system. It includes CBE (Cytosine Base Editors) and ABE (Adenine Base Editors), which use their fused deaminase to modify bases within the editing window, enabling the replacement of single bases through DNA replication or repair. Compared to traditional CRISPR/Cas9 technology, CBE or ABE does not generate DNA double-strand breaks or require donor DNAs and is not dependent on the cell cycle for precise gene repair (Table 1. Comparison of AccuBase™ with other gene editing tools). Most human genetic diseases are caused by single nucleotide mutations, and thus, DNA base editing technology can be broadly applied in the treatment of these genetic diseases. Base editing can also knockout genes by mutating start codons, introducing stop codons, or mutating splice sites.
Table 1. Comparison of AccuBase™ with other gene editing tools
|
CRISPR Nucleases |
BE4max |
AccuBaseTM |
|
|
DNA double-strand break |
Yes |
No |
No |
|
Target Insertion/Deletion |
Yes |
No |
No |
|
Single base editing |
No |
Yes |
Yes |
|
Editing efficiency |
80-100% |
80-100% |
80-100% |
|
Off target; Unwanted INDEL; chromosomal rearrangements |
1%-5% Chromosomal translocation, Large insertion/deletion, abnormalities (1 to 3 gene editing) |
Off target mutations |
Close to zero |
|
Size |
4Kb |
around 5.5Kb |
around 5.5Kb |
Due to the exposure of the deaminase, the traditional base editors are prone to random deamination, leading to off-target effects. This limits the application of base editors. To overcome the off-target problem, Base Therapeutics designed and engineered a new type of base editor, AccuBase™, which maintains high editing activity while significantly reducing the occurrence of off-target effects.
Principle of Gene Knockout with AccuBase™
The commercialized AccuBase™ by KACTUS is a cytosine base editor. This enzyme, when combined with sgRNA (including sgRNA for SpCas9), forms an RNP complex. Once introduced into the cells and bound to the target site, the RNP complex converts the cytosine at the respective position (the editing window is at positions 3-12, with the first position being far from the PAM site) into uracil without generating DNA double-strand breaks, eventually creating uracil to thymine mutation via DNA repair. The C->T mutation can also create gene knockout by generating stop codons or disrupting splicing sites on the target sequence (Figure 1).

Figure 1: Application of AccuBase™ in generating point mutation and gene knockout
High Base Editing Efficiency of AccuBase™
AccuBase™ is widely used in gene knockout in different cell types, with the highest knockout efficiency in T cells nearing 100%. AccuBase™ RNP by electroporation into T cells, followed by flow cytometry detection (Figure 2), shows that at the protein level, the AccuBase™ base editing system can efficiently knock out PD1, B2M, and TRAC proteins in T cells. Among these, the knockout efficiency of PD1 and B2M is above 80%, and the knockout efficiency of TRAC reaches 96%.

Figure 2: Knockout efficiency of AccuBase™ in T cells evaluated by flow cytometry.
Near-to-Zero Off-Target Occurrence with AccuBase™
The ingenious structural design and the inherent characteristics of single-base editing make AccuBase™ an editor with near-to-zero off-target effects compared to other editing tools, revolutionarily solving the issue of safety concern, the biggest pain point in the world gene editing industry.
By utilizing GOTI (genome-wide off-target analysis by two-cell embryo injection) to analyze the off-target levels of AccuBase™ across the genome (Figure 3), it was found that compared to the group using the other editor, (where over 700 SNVs were detected), the SNVs detected within the AccuBase™ group were close to the level of the GFP (negative) control group. These results indicate that the AccuBase™ is of higher safety compared to other gene editing tools.

Figure 3: Comparison of off-target effects by GOTI
By leveraging its Structure Aided Design and Multiplex Screening SAMS™ platform, KACTUS underwent process development for AccuBase™, a series of steps including screening of protein expression system, purification process optimization, and formulation development, and successfully produced DNA base editing proteins with high stability, high purity, and high activity. The manufacturing and filing for AccuBase™ is conducted in strict compliance with GMP standards, meeting the requirements in both the United States and China for clinical project filing. Currently, KACTUS and Base Therapeutics have reached an agreement on the production and sales of the GMP-grade base editor AccuBase™ around the world.
Product Information for AccuBase™
KACTUS provides both Research-Grade and GMP-grade AccuBase™ products on the market, which are currently in stock. Feel free to reach out and request a testing sample today!
|
Product Name |
Catalog |
Size |
|
KD-0001 |
200μg/500μg/1mg |
|
|
GMP-KD-0001 |
1mg |
NOTE: BS-EP1 is product name, AccuBase™ is the commercial name.