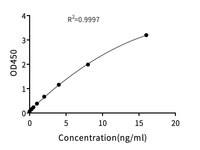
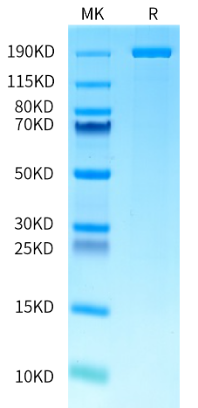
The Rise of Allogeneic Cell Therapies: Strategies, Challenges, and Clinical Advances
By Mallory Griffin
As proteins and enzymes for cell therapy continue to revolutionize the treatment landscape for cancer and hereditary diseases, the field is shifting its focus from personalized, autologous approaches to scalable, off-the-shelf allogeneic solutions. This transition brings new opportunities and inevitable new challenges. Therapeutic developers in the advanced therapy field are actively exploring various options to advance universal cell therapies into clinical stages. This includes using alternative immune cell types and implementing precise gene editing strategies. Here, we discuss an overview of the current trends of allogeneic cell therapy, highlighting the key technology platforms, ongoing clinical programs, and also the gene editing technologies that empower allogeneic cell therapies.
KACTUS is proud to support cell therapy process development and manufacturing with a high-performance base editor and Cas9 enzyme.
Autologous vs. Allogeneic Cell Therapy
Autologous chimeric antigen receptor T-cell (CAR-T) therapy is a “vein-to-vein” live cell therapy, which minimizes the risk of immune rejection. Currently, all FDA-approved CAR-T cell therapies follow the autologous treatment pathway. This involves collecting the patient’s own cells as the starting material, engineering them ex vivo to express the CAR on the cell surface, expanding them, and reinfusing them back into the patient. Some patients, however, cannot benefit from this therapeutic scenario due to difficulties in cell collection or poor cell quality. Additionally, the high manufacturing costs and long production timelines limit the widespread adoption of autologous CAR-T therapy.
Allogeneic CAR-T therapy, on the other hand, is an “off-the-shelf” type of cell therapy. It collects cells from healthy donors to manufacture cell products that can be used for multiple patients in a single production run. Each batch can potentially serve dozens or even hundreds of patients. Compared to autologous therapies, this approach overcomes limitations related to starting material and production time, enabling on-demand usage and offering a feasible solution to the challenges of autologous CAR-T therapy.
Advantages and Challenges of Allogeneic CAR-T cell therapy
Allogeneic CAR-T therapy overcomes the limitation of using autologous cells, offering a new treatment option for patients who cannot provide qualified cells. Its “off-the-shelf” nature shortens the traditional one-month manufacturing timeline of autologous therapies, offering on-demand availability. Additionally, large-scale production can significantly reduce the final costs and increase accessibility.
However, allogeneic cell therapy also faces certain limitations. The major risks for allogenic therapy stem from host-mediated graft rejection and graft-versus-host disease (GvHD). The host immune system recognizes donor cells’ MHC molecules and rapidly eliminates the CAR-T cells. This shortens the persistence of CAR-T cells in the patient’s body, leading to reduced efficacy. Moreover, donor CAR-T cells will also attack host tissues, causing symptoms in the skin, liver, and gastrointestinal tract, and potentially leading to multi-organ damage that can be life-threatening. Fortunately, these risks can be effectively minimized through genetic engineering of cell products (Figure 2) or by using other types of immune cells (Table 1). These cell types offer unique immunological features that can reduce the need for extensive gene editing or avoid GvHD at the source.
![Genetic modifications required for various off-the-shelf CAR cell products. Nat Rev Clin Oncol 22, 10–27 (2025) [1].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/CellTherapy_Edits_Blog.png?v=1744032007)
Figure 2. Genetic modifications required for various off-the-shelf CAR cell products. Nat Rev Clin Oncol 22, 10–27 (2025) [1].
|
Alternative Cell Type |
Potential Advantages |
Potential Disadvantages |
Representative Companies & Pipelines |
|
γδ T Cells |
Low risk of GvHD, no need to disrupt TCR, may require less gene editing |
Represent only a small fraction of peripheral T cells; difficult to isolate and expand; uncertain efficacy compared to CAR-T therapies |
Adicet Bio, ADI-001 (targets CD20) |
|
UCB-Derived T Cells |
Relatively easy to obtain, no need to collect peripheral blood lymphocytes |
Requires HLA matching |
UCELLO, UC101 (targets CD19) |
|
NK Cells |
Low GvHD risk, abundant in peripheral and cord blood, multiple cytotoxic mechanisms, lower toxicity in current clinical data |
Prone to exhaustion, difficult to transduce, risk of rejection, short-lived without cytokine support |
Wugen, WU-NK-101 |
|
iPSC-Derived Cell Types |
Can differentiate into various cell types, more amenable to multiplex editing and storage |
Immature cell phenotypes may limit efficacy |
Fate Therapeutics, FT819 (targets CD19) |
Table 1. Alternative cell types for CAR-based cell therapy
Clinical Advances of Gene Editing Fueling Allogeneic Cell Therapy
While selecting the right cell type is critical for minimizing GvHD risk and enhancing compatibility, gene editing serves as a more powerful strategy to fine-tune these cells for therapeutic use. Regardless of the starting cell—whether T cells, NK cells, or iPSC-derived cells—precise genome modifications can eliminate immunogenicity, enhance function, and support large-scale, off-the-shelf manufacturing. Technologies such as CRISPR-Cas9, base editing, prime editing, and epigenetic editing are accelerating the development of universal cell therapies and are now advancing into clinical trials. Supporting these technologies often requires specialized custom protein expression to produce engineered enzymes or tailored gene editing components that meet precise research and clinical specifications. This marks a new era in engineered cell therapies for cancer, genetic disorders, and infectious diseases. Examples include:
-
Beam Therapeutics has initiated a Phase I/II clinical trial for Beam-201, a CAR-T therapy targeting CD7 in T-cell leukemia and lymphoma. The therapy uses base editing to knock out the expression of CD7, TRAC, CD52, and PDCD1 on T cells, aiming to enhance the allogeneic compatibility of CAR-T cells [2].
-
Base Therapeutics developed NK510, a gene-edited NK cell therapy using single-base editing technology. In 2024, NK510 received clinical approvals in the U.S. for the treatment of advanced solid tumors. The therapy uses the protein-based base editor Accubase® to edit NK cells in vitro [3].
-
On April 9, 2024, Prime Medicine announced that its prime editing therapy for treating chronic granulomatous disease (CGD) has received FDA approval for an IND application and will proceed to a global Phase 1/2 clinical trial [4].
-
In November 2024, Epigenetic Medicine received CTA approval from New Zealand’s Medsafe and the Health and Disability Ethics Committees (HDEC) for EPI-003, an epigenetic editing therapy targeting chronic hepatitis B caused by HBV (5).
|
Company |
Products |
CAR |
Cell Source |
Gene-editing Tools |
Gene-editing Strategy |
Phase |
|
P-BMCA-ALL01 |
BCMA |
T cell |
Cas-CLOVER |
KO TRBC and B2M |
Phase I |
|
|
P-CD19CD20-ALL01 |
CD19/CD20 |
Phase I |
||||
|
cema-cel |
CD19 |
T cell |
TALEN |
KO TRAC and CD52 |
Phase II |
|
|
ALLO-316 |
CD70 |
Phase I |
||||
|
WU-CART-007 |
CD7 |
T cell |
CRISPR |
KO TCR and CD7 |
Phase I |
|
|
WU-NK-101 |
/ |
NK cell |
N/A |
N/A |
Phase I |
|
|
ADI-001 |
CD20 |
γδT cell |
N/A |
N/A |
Phase I |
|
|
CB-010 |
CD19 |
T cell |
CRISPR |
KO TRAC, PD1 |
Phase I |
|
|
UCART22 |
CD22 |
T cell |
TALEN |
KO TRAC and CD52 |
Phase I/II |
|
|
UCART20×22 |
CD20 and CD22 |
KO TRAC and CD52 |
Phase I/II |
|||
|
CYAD-211 |
BCMA |
T cell |
shRNA knockdown of TCR |
CD3ζ |
Phase I |
|
|
CYAD-101 |
NKG2D |
TCR inhibitory molecule (TIM) |
CD3ζ |
Phase I |
||
|
CTX112 |
CD19 |
T cell |
CRISPR/Cas9 |
KO TRAC and B2M |
Phase I |
|
|
CTX131 |
CD70 |
Phase I |
||||
|
FT-819 |
CD19 |
iPSC-derived T cell |
CRISPR/Cas9 |
KO TRAC |
Phase I |
|
|
FT522 |
CD19 |
iPSC-derived NK cell |
CRISPR/Cas9 |
KO CD38 |
Phase I |
|
|
Beam Therapeutics |
Beam-201 |
CD7 |
T cell |
Base editing |
KO CD7, TRAC, CD52 and PD1 |
Phase I |
|
BRL-301 |
CD19 |
T cell |
CRISPR/Cas9 |
not disclosed |
Phase I |
|
|
Bioheng Therapeutics |
CTD402 |
CD7 |
T cell |
CRISPR/Cas9 |
KO CD7, TCR, etc., |
Phase I |
|
Ucello Biotechnology |
UC101 |
CD19 |
UCB-T cell |
N/A |
N/A |
Phase I |
Table 2. Several universal (allogeneic) cell therapy pipelines currently in clinical stages.
KACTUS’ Gene Editing Toolbox Accelerates the Development of Universal Cell Therapies
KACTUS is committed to providing high-quality genome editing tools for cell therapy companies. Backed by a certified quality management system and nearly 10,000 square meters of GMP manufacturing facilities, KACTUS has built a comprehensive product portfolio featuring a range of high-performing gene editing enzymes.
Among them, GMP-grade Cas9 protein has already been used by nearly 100 biopharmaceutical companies, supporting end-to-end needs from early research to clinical IND filings. In addition, AccuBase® Base Editor, a proprietary cytosine base editor developed by Base Therapeutics and licensed to KACTUS for GMP manufacturing and commercialization, has already advanced into the clinic with an IND application approved by the FDA.
Contact us to learn more about our gene editing enzymes or request a quote. For teams working on engineered TCR-T or CAR-T therapies, KACTUS also offers soluble TCR expression services to support TCR screening, validation, and downstream functional studies.
Browse Products
CRISPR Cas9 Protein, GMP-Grade
CRISPR Cas9 Protein, Research-Grade
Cas9 Protein ELISA Kit, Research-Grade
AccuBase® Cytosine Base Editor, GMP-Grade
AccuBase® Cytosine Base Editor, Research-Grade
AccuBase Cytosine Base Editor ELISA Kit, Research-Grade