For Better Symptom Relief: Targeted Therapy for Asthma
By Mallory Griffin
Asthma is a non-specific chronic lung disease caused by inflammation around the airways and muscle tightening, leading to breathing difficulties. It is characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and coughing, often occurring or worsening at night or in the early morning. These episodes significantly affect the patient's quality of life. The development of asthma is mainly influenced or triggered by genetics, individual constitution, and allergens.
![During an asthma attack, the airways become swollen and filled with mucus [1].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/Asthma_Fig1.png?v=1744636971)
Figure 1. During an asthma attack, the airways become swollen and filled with mucus [1].
Based on immune-inflammatory mechanisms and biomarkers, asthma can be classified into Type 2 and non-Type 2 asthma:
Type 2 asthma is primarily driven by Th2-type immune responses and is often triggered by allergens, pollutants, or microbes. These substances are captured by dendritic cells, leading bronchial epithelial cells to release IL-25, IL-33, and TSLP. These cytokines activate type 2 innate lymphoid cells (ILC2s), which play a crucial role in initiating the type 2 immune response. Type 2 asthma includes allergic asthma and eosinophilic asthma. Elevated levels of Th2-related cytokines such as IL-4, IL-5, IL-13, and IgE are hallmark features of Type 2 asthma. More than 50% of adult asthma cases are classified as Type 2.
Non-Type 2 asthma lacks well-defined biomarkers but involves a variety of cytokines, including IL-17A, IL-6, IL-8, and IL-1β, which induce different types of inflammation such as Th1 and Th17 responses. The mechanisms of non-Type 2 asthma are more complex and include multiple inflammatory and non-inflammatory pathways, such as neutrophilic inflammation, Type 1 immune responses, Type 3 immune responses (IL-17-mediated), systemic inflammatory responses, metabolic abnormalities, and neuro-immune mechanisms. Non-Type 2 asthma often responds poorly to glucocorticoid treatment.

Figure 2. Mechanisms of Type 2 and Non-Type 2 Asthma [1].
Traditional therapies that focus on symptom control are often ineffective for a significant subset of patients, especially those with moderate-to-severe asthma or those who are unresponsive to or dependent on steroids. With advances in our understanding of asthma mechanisms, effective and safe molecular targeted therapies can act on specific inflammatory pathways or biomarkers, enabling more precise treatment. These therapies can significantly reduce the frequency of acute exacerbations, decrease reliance on oral steroids, and ultimately improve quality of life.
Based on the currently defined asthma subtypes, therapeutic targets can be broadly categorized as follows:
Classic Inflammatory Factors and Receptors (IgE, IL-5 and IL-5R, IL-4 and IL-4Rα, etc.)
These targets play key roles in the Type 2 inflammatory pathway. IgE is a central mediator of allergic responses—it binds to IgE receptors on mast cells and eosinophils (EOS), triggering degranulation and the release of inflammatory mediators. Omalizumab, the first biologic targeting IgE and the first targeted therapy for asthma, was approved in Australia in 2002. Omalizumab specifically binds to circulating IgE, significantly lowering free serum IgE levels and reducing the frequency of acute exacerbations in patients with moderate to severe allergic asthma.
IL-5 and IL-5R stimulate eosinophil activation and proliferation, leading to the release of inflammatory factors, mucus secretion, and airway smooth muscle hypertrophy—making them key drivers of eosinophilic asthma. Approved drugs targeting IL-5 include Mepolizumab and Reslizumab, while Benralizumab targets IL-5R. These therapies block the interaction between IL-5 and its receptor or eliminate eosinophils via antibody-dependent cellular cytotoxicity (ADCC). Their clinical use requires precise patient selection based on blood eosinophil counts, and they are less effective in IL-5–independent cases.
IL-4 and IL-4Rα are major drivers of Type 2 immune responses and IgE-mediated allergic reactions. They directly induce airway mucus secretion and structural remodeling and are more strongly associated with early-onset and allergic asthma. Dupilumab, a monoclonal antibody targeting IL-4Rα, inhibits both the IL-4 and IL-13 pathways. It is highly effective in treating moderate to severe eosinophilic or steroid-dependent asthma, particularly in patients with coexisting atopic conditions such as atopic dermatitis or nasal polyps.
Upstream Inflammatory Pathways: TSLP and IL-33
Targeting upstream factors aims to block the early initiation of the inflammatory response, enabling broader control. These pathways often involve epithelial barrier–derived inflammatory signals.
TSLP (Thymic Stromal Lymphopoietin) is an epithelial-derived alarmin secreted in response to environmental stimuli (e.g., viruses, allergens). It activates dendritic cells and other immune cells to initiate a Th2-type immune response, contributing to the development of airway inflammation in asthma. Targeting TSLP offers a strategy to intercept the inflammatory cascade at its origin.

Figure 3. TSLP Signaling Pathway [2].
Tezepelumab (brand name Tezspire), the first antibody drug targeting TSLP, was approved for the market in December 2021. It is effective not only in patients with high Type 2 inflammation but also in those with low Type 2 inflammation, making it the first asthma biologic that does not rely on specific biomarkers. Since its launch, Tezepelumab has generated several hundred million dollars in annual sales, with continued growth.
The success of Tezepelumab has sparked a surge in TSLP-targeted drug development, with innovations in both molecular formats and routes of administration. Among the more advanced candidates is Bosakitug, Biosion’s independently developed TSLP inhibitor featuring a bispecific antibody design, currently in Phase 3 clinical trials for asthma. Inhaled formulations such as AZD-8630 (developed by AstraZeneca and Amgen) and LQ043H (developed by Luoqi Bio) aim to greatly improve patient compliance in asthma treatment.
IL-33 is an inducer of Type 2 adaptive immunity. It is passively released following tissue damage and signals through the IL-1 receptor accessory protein ST2, promoting the production of chemokines and cytokines associated with Type 2 inflammation. IL-33 also plays a crucial role in the survival and activation of eosinophils. Elevated levels of IL-33 have been observed in airway biopsies from patients with severe asthma.
Tozorakimab, developed by AstraZeneca, demonstrated a significant 43% reduction in exacerbation rates in a key Phase 2 trial for eosinophilic asthma, with about 50% reduction observed in patients with high IL-33 levels. It also showed marked improvement in FEV1. In contrast, Itepekimab, jointly developed by Regeneron and Sanofi, reduced peripheral blood eosinophil counts but failed to significantly improve exacerbation rates or lung function, missing its primary endpoints and has since been discontinued. Moving forward, IL-33-targeted therapies may require more precise patient stratification—such as based on IL-33 or ST2 levels and eosinophil counts—to improve response rates.

Table 1. Approved Biologic Therapies for Asthma [3].
Targets Focused on Low Type 2 and Non-Type 2 Asthma, such as IL-17, IL-6, TNF-α
These factors mainly mediate neutrophil-driven inflammation and are involved in steroid-resistant asthma.
IL-17, secreted by Th17 cells, can directly stimulate airway epithelial cells to produce chemokines such as CXCL1 and CXCL8, recruiting neutrophils, promoting mucus secretion, macrophage mobilization, and airway smooth muscle reactivity. These effects are particularly evident in adults with severe or steroid-insensitive asthma [4]. However, monoclonal antibodies against IL-17A, such as Secukinumab and Brodalumab, although showing some improvements in certain indicators, did not produce statistically significant differences in ACQ scores, lung function, or SABA usage.
IL-6 overexpression is linked to neutrophil activation and can cooperate with TGF-β to promote the differentiation of naïve T cells into Th17 cells, intensifying IL-17–mediated inflammation. The IL-6 monoclonal antibody Tocilizumab showed partial improvement in lung function in a Phase 2 trial for asthma, but has not yet been widely adopted.
TNF-α activates endothelial and epithelial cells, promotes expression of adhesion molecules like ICAM-1, enhances neutrophil migration, directly stimulates airway smooth muscle contraction, and induces neurogenic inflammation. Etanercept, a TNF-α receptor fusion protein, showed some efficacy in early clinical trials for steroid-resistant asthma, but was discontinued due to side effects such as infection.
It is evident that clinical failures for non-Type 2 asthma drugs are more common, likely due to the high heterogeneity, lack of clear biomarkers, and possible compensatory mechanisms across pathways.
Different targets represent distinct mechanisms and stages of asthma pathogenesis. Given the complexity of asthma, with numerous proteins involved, blocking a single target usually achieves partial efficacy. Combination therapy is expected to become a key trend in future asthma treatment. Additionally, more precise patient stratification, improved drug delivery methods, and novel therapies—such as gene therapy, mesenchymal stem cell therapy, extracellular vesicle-based treatments, and T-cell therapy—may pave the way for breakthroughs. These advancements have the potential to shift asthma treatment from symptom control to precise intervention, and possibly even curative approaches.
| Target | Name | Aliases | Type | Company | Highest clinical stage for asthma |
|
IL-5
|
610 | NA | mAb | Sunshine Guojian |
Phase 3
|
| SHR-1703 | NA | mAb | Hengrui | ||
| GSK3511294 | AQ82742999, GSK 294, Depemokimab | mAb | GSK | Phase 1 | |
|
IL-4R
|
MG-K10 | Comekibart | mAb | Mabgeek, China Medical System |
Phase 3
|
| CM310 | Stapokibart | mAb | KeyMed, CSPC ZhongQi | ||
| SIM0718 | CBP-201, Rademikibart | mAb | Simcere, Connect | ||
| LQ-036 | NA | Nanobody | Novamab |
Phase 2
|
|
| GR1802 | Telikibart | mAb | Genrix | ||
|
IL-13
|
APG777 | NA | mAb | Apogee | Phase 1 |
| BSI-045B | TQC2731, Bosakitug | mAb | Biosion, Chia Tai Tianqing | Phase 3 | |
| HBM9378 | A-378, SKB-378, WIN-378 | mAb | Harbour, Kelun-Biotech |
Phase 2
|
|
| SHR-1905 | AIO-001, GSK-5784283 | mAb | Hengrui, GSK | ||
| CM326 | NA | mAb | KeyMed, CSPC Zhongqi | ||
| AZD-8630 | AGM104 | Ab. fragment | AstraZeneca, Amgen | ||
|
TSLP
|
MK-8226 | NSI-8226, Solrikitug | mAb | Uniquity | |
| QX008N | NA | mAb | Qyuns |
Phase 1
|
|
| LQ043H | NA | sdAb | Novamab | ||
| GB-0895 | NA | mAb | Generate | ||
| STSA-1201 | NA | mAb | Staidson | ||
| APG333 | NA | mAb | Apogee | ||
| GR2002 | NA | Dual-Epi BsAb | Genrix | ||
| TSLPR | UPB-101 | ASP-7266, Verekitug | mAb | Upstream, Astellas | Phase 2 |
| IL-33 | MEDI3506 | Tozorakimab | mAb | AstraZeneca | Phase 2 |
| ST2 | RG6149 | AMG 282, MSTT 1041 A, RO7187807, Astegolimab | mAb | Roche, Amgen | Phase 2 |
| IL-25 | XKH001 | NA | mAb | Kanova | Phase 1 |
| IL-5, IL-4R | RC1416 | NA | BsNb | Regenecore | Phase 1 |
| IL-4Rα, TSLP | IBI-3002 | NA | BsAb | Innovent | Phase 1 |
| IL-13, TSLP | SAR-443765 | Lunsekimig | BsNb | Sanofi | Phase 2 |
| IL-11, TSLP | HB0056 | NA | BsAb | Huaota | Phase 1 |
Table 2. Asthma therapies currently in clinical development.
Recombinant Proteins for Asthma-Based Drug Discovery
Recombinant target proteins are the cornerstone of targeted drug development, playing a critical role throughout the entire process—from target discovery and antibody optimization to efficacy evaluation. KACTUS offers a wide range of asthma-related target proteins, all rigorously quality-tested and suitable for diverse applications such as immunological assays and diagnostic development.
Example Product Data
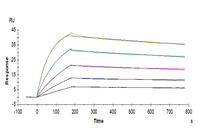
Human TSLP Protein (TSP-HM401)

Immobilized Human TSLP, His Tag at 1ug/ml (100ul/well) on the plate. Dose response curve for Anti-TSLP Antibody, hFc Tag with the EC50 of 13.0ng/ml determined by ELISA.

Human TSLP, His Tag captured on CM5 Chip via Anti-His Antibody can bind Human TSLPR, hFc Tag with an affinity constant of 1.04 nM as determined in SPR assay (Biacore T200).
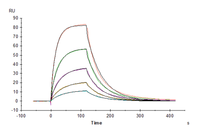
Human IL-4 R alpha/CD124 Protein (ILA-HM14R)

Immobilized Human IL-4 R alpha, His Tag at 1 ug/ml (100 ul/Well). Dose response curve for Biotinylated Human IL-4, His-Avi Tag with the EC50 of 11.2 ng/ml determined by ELISA (QC Test).

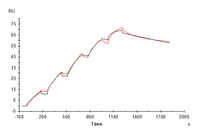
Human IL-17R alpha, His Tag immobilized on CM5 Chip can bind Human IL-17A, His Tag with an affinity constant of 1.57 nM as determined in SPR assay (Biacore T200).
Product List
References
[1] https://en.wikipedia.org
[2] Role of thymic stromal lymphopoietin in allergy and beyond. Nat Rev Immunol. 2023 Jan;23(1):24-37. doi: 10.1038/s41577-022-00735-y.
[3] Precision medicine for severe asthma - Biological targeted therapy. Int Immunopharmacol. 2024 Jun 15;134:112189. doi: 10.1016/j.intimp.2024.112189.
[4] TH17 cells and corticosteroid insensitivity in severe asthma. J Allergy Clin Immunol 2022; 149: 467–479. doi:10.1016/j.jaci.2021.12.769
[5] Real-World Experience with Dupilumab in Severe Asthma: One-Year Data from an Italian Named Patient Program. J Asthma Allergy. 2021 May 27;14:575-583. doi: 10.2147/JAA.S312123.
[6] Targeting of TSLP and IL-13 by the Novel NANOBODY® Molecule SAR443765 Reduces FeNO in Asthma Following Single Dose Exposure. doi: 10.1164/ajrccm-conference.2023.207.1_MeetingAbstracts.A6816
[7] Advances in non-type 2 severe asthma: from molecular insights to novel treatment strategies. Eur Respir J. 2024 Aug 15;64(2):2300826. doi: 10.1183/13993003.00826-2023.
[8] Approach to non-type 2 asthma. Respir Med. 2023 Sep;216:107327. doi: 10.1016/j.rmed.2023.107327.
[9] Novel asthma treatments: Advancing beyond approved novel step-up therapies for asthma. Ann Allergy Asthma Immunol. 2025 Jan;134(1):9-18. doi: 10.1016/j.anai.2024.09.016. Epub 2024 Oct 10.