At the Crossroads of Metabolic and Cancer Therapeutics — GDF15
By Mallory Griffin
In March 2025, GenFleet Therapeutics announced that its independently developed bispecific antibody GFS202A, targeting GDF15 and IL-6, received approval from the National Medical Products Administration (NMPA) for clinical trials aimed at pre-cachexia and cachexia in cancer patients. This marks the world’s first bispecific antibody targeting both GDF15 and IL-6 and comes after Pfizer completed a Phase 2 clinical trial in September for its GDF15 antibody drug, Ponsegromab.
GDF15 is increasingly recognized as a key target in cancer cachexia and is also closely associated with various metabolic diseases. This unique positioning—where GDF15 contributes to both muscle wasting in cancer patients and metabolic regulation in obesity and diabetes—has placed it at the intersection of two major therapeutic areas. As research continues to uncover its context-dependent roles, GDF15 stands out as a compelling but complex target in both oncology and metabolic drug development.
Recombinant GDF15 proteins for Research
To support GDF15 drug development, KACTUS provides a catalog of bioactive GDF15 and GFRAL proteins expressed from HEK293 or E. coli expression systems.
GDF15 is a stress response factor
GDF15 (Growth Differentiation Factor 15) belongs to the TGFβ superfamily of proteins. Its primary role in the human body is a metabolic and stress signal molecule, involved in regulating energy balance, appetite, and inflammatory responses. These stress signals include hypoxia, mitochondrial dysfunction, metformin, and endurance exercise. Elevated levels of GDF15 are observed in conditions such as obesity, type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cardiovascular diseases (such as heart failure), and cancer, making it a potential therapeutic target for these diseases.
GDF15 is initially synthesized as a biologically inactive precursor protein (pre-pro-GDF15). The N-terminal signal peptide of the precursor is essential for its transport and secretion and is cleaved off to produce pro-GDF15 (~30 kDa). Pro-GDF15 is then further cleaved at the C-terminus to generate the mature form of GDF15 (~13 kDa). Mature GDF15 forms a homodimer via disulfide bonds, which is the predominant secreted form found in serum.
![Figure 1. Synthesis, Maturation, and Secretion of GDF15 [1].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GDF15_Synthesis.jpg?v=1743086010)
Figure 1. Synthesis, Maturation, and Secretion of GDF15 [1].
GDF15 in Obesity and Diabetes
GDF15 binds to its specific receptor GFRAL in the brainstem and activates downstream signaling pathways such as PI3K-AKT through the GDF15-GFRAL-RET axis. This leads to reduced food intake and lower blood glucose levels, thereby helping to maintain energy homeostasis.
![Figure 2. Mechanism of GDF15-Mediated Suppression of Energy Intake [2].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GDF15_Blog_2.png?v=1743086018)
Figure 2. Mechanism of GDF15-Mediated Suppression of Energy Intake [2].
Obesity and diabetes are closely associated with GDF15. In obese patients, GDF15 helps regulate metabolism by suppressing appetite and improving metabolic function. However, due to receptor desensitization or signaling resistance in these patients, the physiological effects of GDF15 tend to be weak. In diabetes, GDF15 can directly alleviate disease progression by enhancing insulin sensitivity. Therefore, for both obesity and diabetes, the therapeutic goal is to amplify GDF15’s effects on promoting lipolysis and glucose metabolism.
The GDF15-related drug modality are often GDF15 analogs or fusion proteins. Representative clinical candidates include CIN-109 and YH-34160. CIN-109, co-developed by Janssen Sciences Ireland UC (a subsidiary of Johnson & Johnson) and CinFina Pharma, is a novel long-acting GDF15 analog. In obese patients, CIN-109 reduced food intake by approximately 50% within 1–2 months of administration, accompanied by around 3.7% weight loss. YH-34160, an engineered GDF15 variant-Fc fusion protein developed by Yuhan Corporation, has already passed an Investigational New Drug (IND) review.
GDF15 and Cancer Cachexia
As a hidden killer in cancer treatment, cancer cachexia—with its high mortality rate, therapeutic complexity, and broad impact on prognosis—demands that both clinicians and researchers treat it as a distinct disease requiring targeted intervention. Cancer cachexia is highly prevalent among cancer patients and is characterized by severe weight loss and reduced appetite, significantly affecting patient survival and tolerance to cancer therapies.
GDF15 is a key driver of cancer cachexia, capable of suppressing appetite and promoting metabolic dysfunction. Studies have found that GDF15 levels are significantly elevated in the serum of cancer patients [3], and this increase is associated with weight loss and reduced BMI. Therefore, unlike in obesity and diabetes where GDF15 activity may be enhanced, alleviating cachexia requires inhibiting GDF15. Moreover, chemotherapeutic agents such as cisplatin can induce anorexia and worsen cachexia by increasing GDF15 expression. Neutralizing GDF15 has been shown to relieve these symptoms in both mice and non-human primates [4].
Pfizer’s GDF15-targeting antibody drug, Ponsegromab (PF-06946860), demonstrated significant weight gain across all treatment groups after 12 weeks in a Phase 2 clinical trial. Notably, the 400 mg group showed improvements not only in appetite and cachexia-related symptoms but also in physical activity levels and skeletal muscle index.
![Figure 3. Ponsegromab Significantly Increases Body Weight in Cachexia Patients [5].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GDF15_Fig3.png?v=1743086018)
Figure 3. Ponsegromab Significantly Increases Body Weight in Cachexia Patients [5].
In addition to cachexia, GDF15 can inhibit T cell infiltration into the tumor microenvironment (TME) and contribute to resistance to other immune checkpoint inhibitors. Recent studies have found that inhibiting GDF15 with Visugromab (CTL-002) can reverse resistance to the PD-1 antibody Nivolumab [6]. Visugromab is CatalYm’s lead pipeline asset, developed to neutralize GDF15 and relieve immune suppression. It has shown durable anti-tumor activity across multiple solid tumors, demonstrating significant therapeutic potential.
![Figure 4. The Role of GDF15 in Cancer [7]. ](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GDF15_Fig4.png?v=1743086018)
Figure 4. The Role of GDF15 in Cancer [7].
NGM-120 is also advancing to Phase 2 clinical trials for the treatment of hyperemesis gravidarum, based on GDF15’s regulation of the brainstem vomiting center. During pregnancy, approximately 90% of maternal circulating GDF15 is secreted by fetal placental tissues, ultimately leading to nausea similar to that caused by chemotherapy drugs.
Summary of Drugs Targeting GDF15 for Cancer or Cancer Cachexia
|
Drug |
Type |
Company |
Clinical stage |
Conditions |
|
Ponsegromab |
mAb |
Pfizer |
Phase 2 |
Cachexia |
|
Visugromab |
mAb |
CatalYm GmbH |
Phase 2 |
Cancer |
|
Visugromab |
mAb |
CatalYm GmbH |
Preclinical |
Cachexia |
|
NGM-120 |
mAb |
NGM Bio |
Phase 1 |
Cachexia |
|
GFS202A |
BsAb |
GenFleet |
Phase 1 |
Cachexia |
|
AV-380 |
mAb |
Aveo Oncology |
Phase 1 |
Cachexia |
Table 1. Drugs Targeting GDF15 for Cancer or Cancer Cachexia.
GDF15 exhibits two distinct roles. On one hand, it suppresses appetite and improves obesity and insulin resistance; on the other hand, chronic overexpression may lead to muscle wasting, as seen in cachexia. This dual nature is also evident in tumor progression—GDF15 can induce tumor cell apoptosis and exert anti-tumor effects in early stages, but in later stages, it promotes tumor immune evasion and metastasis [8]. This "angel and demon" paradox highlights GDF15’s unique spatial-temporal regulation and raises the bar for precision in GDF15-targeted therapies.
GDF15 and GFRAL Proteins for Drug Development Research
KACTUS offers independently developed recombinant GDF15 proteins expressed in both prokaryotic and eukaryotic systems, overcoming the challenge of low solubility in prokaryotic expression. In addition, a wide range of GFRAL proteins are available, covering multiple species and tag types. All products have been validated for bioactivity, showing high biological activity to support and accelerate drug development efforts.
Product Validation Data

Human GDF15 Protein (GDF-HE115)
Figure 5. Immobilized Human GDF15, His Tag at 0.5 μg/ml (100 μl/well) on the plate. Dose response curve for Human GFRAL, hFc Tag with the EC50 of 13.1 ng/ml determined by ELISA (QC Test).

Human GDF15 Protein (GDF-HE115)
Figure 6. Serial dilutions of Anti-GDF15 Antibody were added into Human GDF15, His Tag : Biotinylated Human GFRAL, His Tag binding reactions. The half-maximal inhibitory concentration (IC50) is 40.8 ng/ml.

Human GFRAL/GFR alpha-like Protein (GFL-HM201)
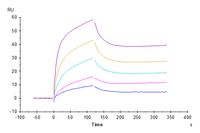
Figure 7. Human GFRAL, hFc Tag captured on CM5 Chip via Protein A can bind Human GDF15, His Tag with an affinity constant of 0.014 nM as determined in SPR assay (Biacore T200).
Product List
|
Protein |
Catalog No. |
Product Description |
|
GDF15 |
||
|
Biotinylated Human GDF15 (H202D) (Primary Amine Labeling), hFc Tag |
||
|
Biotinylated Cynomolgus GDF15 (Primary Amine Labeling), hFc Tag |
||
|
GFRAL |
||
To request a quote, please contact us.
References
[1] Overview of growth differentiation factor 15 (GDF15) in metabolic diseases. Biomed Pharmacother. 2024 Jul;176:116809. doi: 10.1016/j.biopha.2024.116809.
[2] GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021 Oct;17(10):592-607. doi: 10.1038/s41574-021-00529-7.
[3] Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss. Oncol Lett. 2016 Nov;12(5):4219-4223. doi: 10.3892/ol.2016.5183.
[4] GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell Metab. 2020 Dec 1;32(6):938-950.e6. doi: 10.1016/j.cmet.2020.10.023.
[5] Ponsegromab for the Treatment of Cancer Cachexia. N Engl J Med. 2024 Dec 19;391(24):2291-2303.
[6] Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours. Nature. 2025 Jan;637(8048):1218-1227. doi: 10.1038/s41586-024-08305-z.
[7] Macrophages as a Source and Target of GDF-15. Int J Mol Sci. 2024 Jul 3;25(13):7313. doi: 10.3390/ijms25137313.
[8] Pathway in Health and Metabolic Disease: Friend or Foe? Annu Rev Physiol. 2021 Feb 10;83:127-151. doi: 10.1146/annurev-physiol-022020-045449.