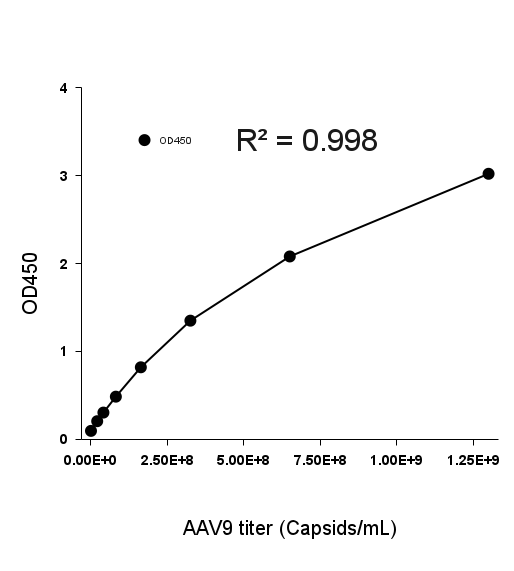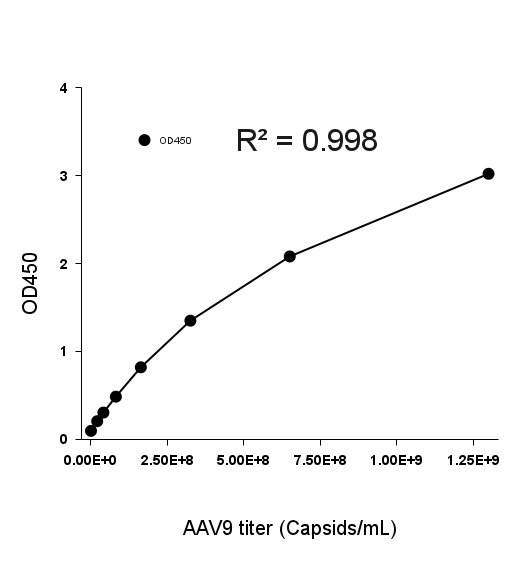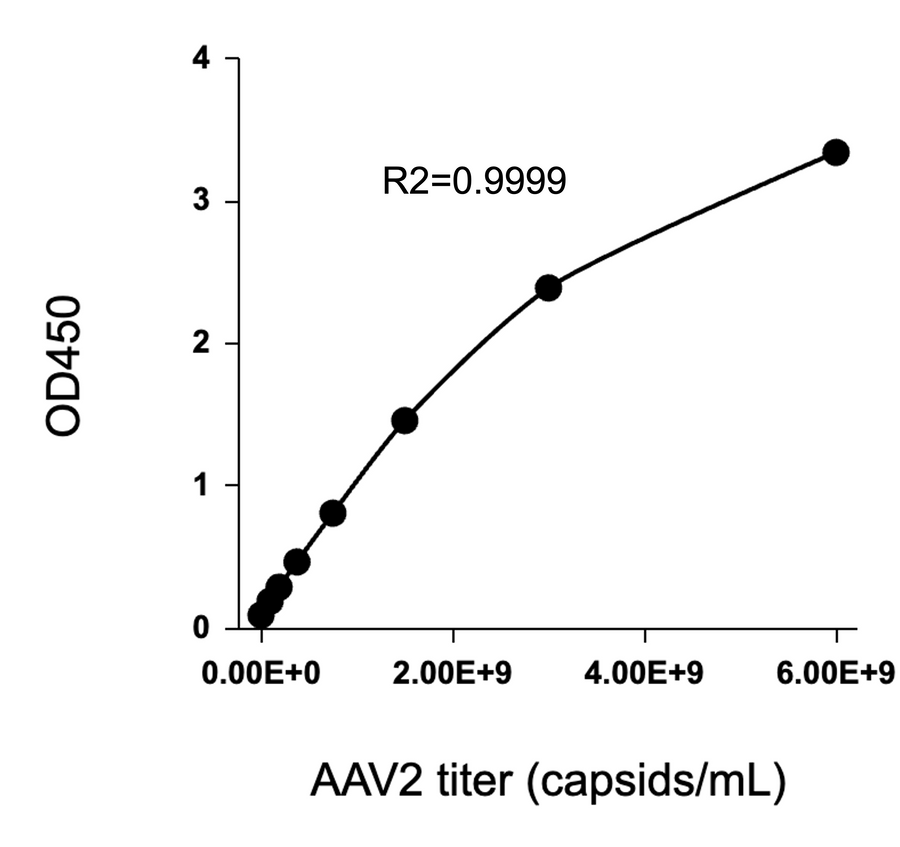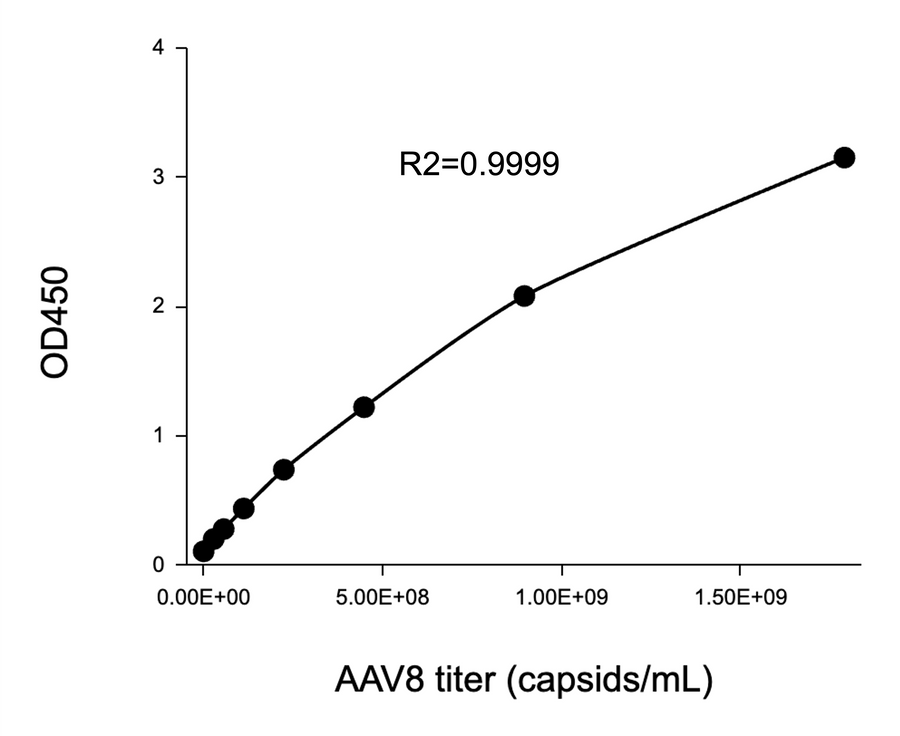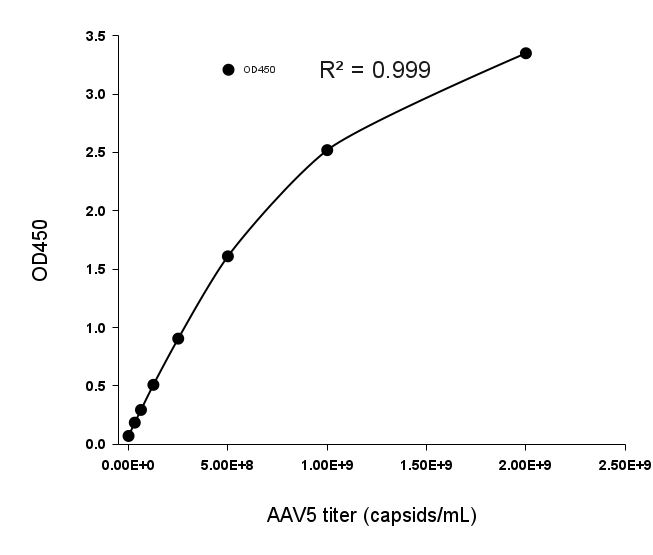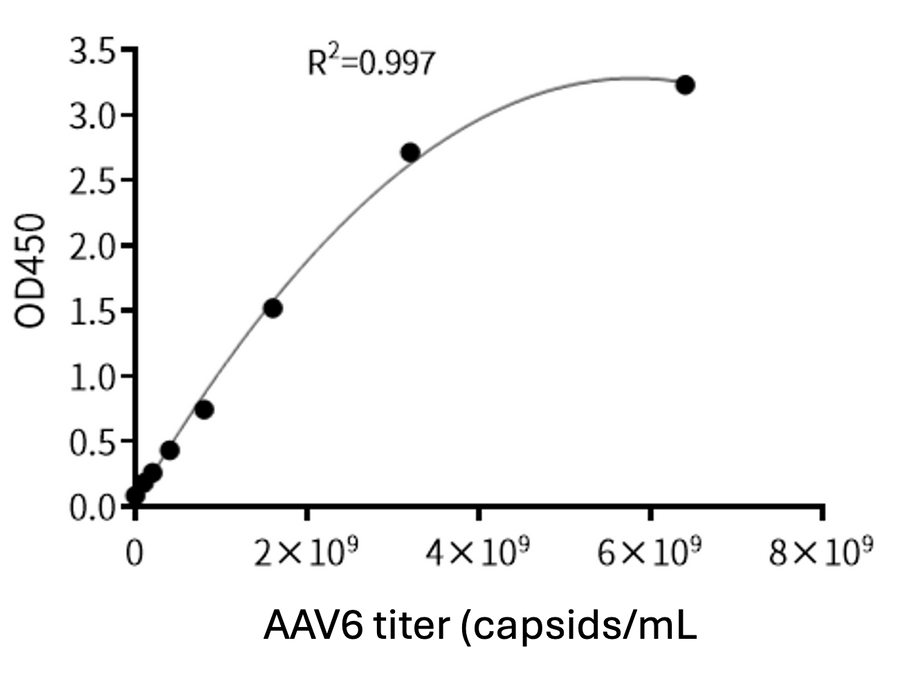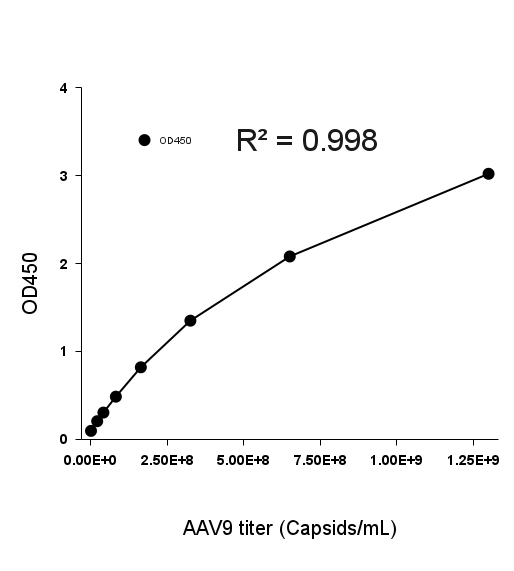
- Description: Quantification of full and empty AAV9 capsids via ELISA.
- Applications: Intact AAV Wild Type Virions
AAV Recombinant Virions
Assembled AAV Virions
Intact Empty AAV Capsids - Quality:
Detection Range: 2.03E+07 capsids/mL - 1.30E+09 capsids/mL
Sensitivity: 1.02E+07 capsids/mL