- Express System: HEK293
- Product Tag: C-His-Avi
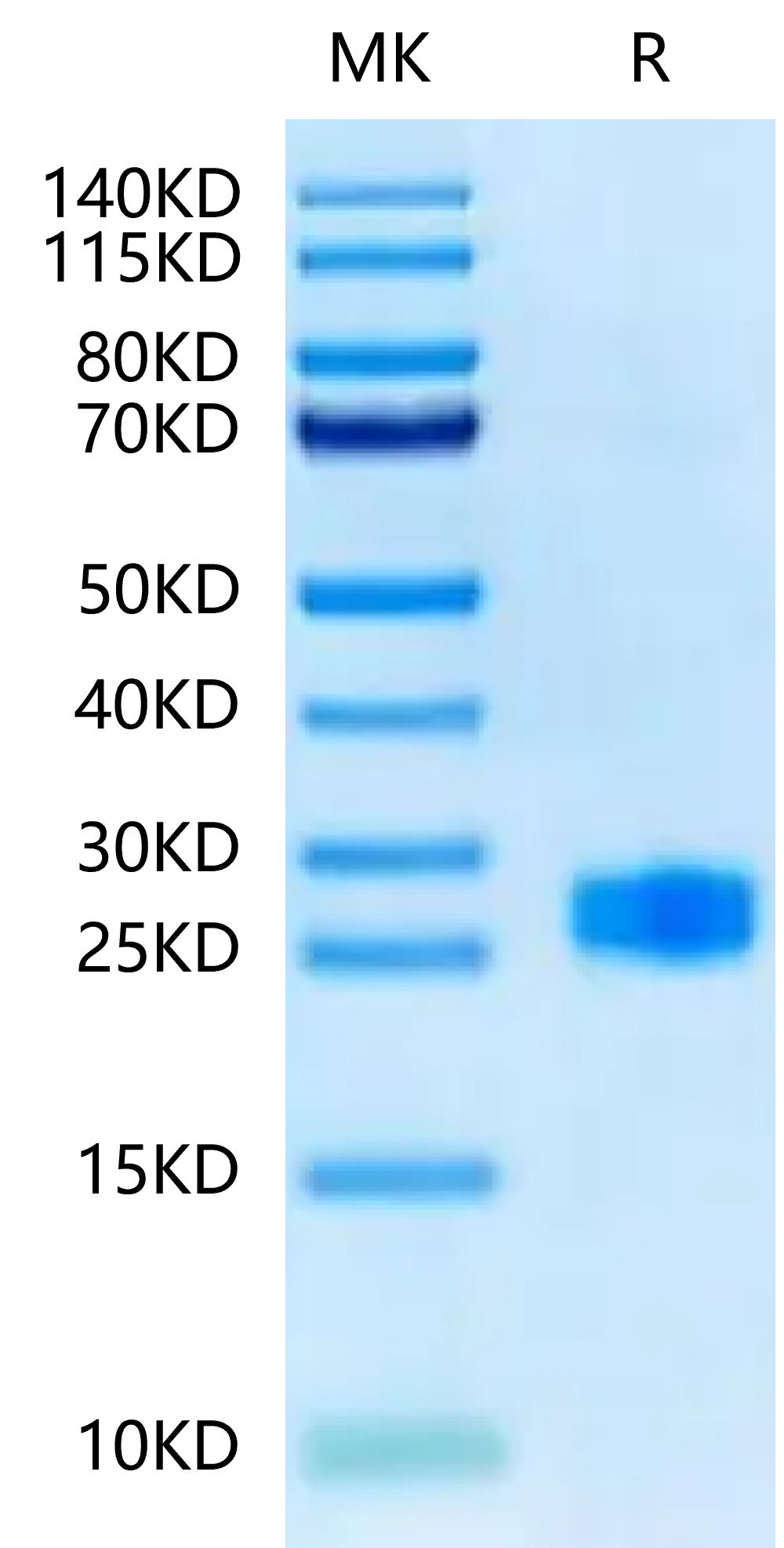
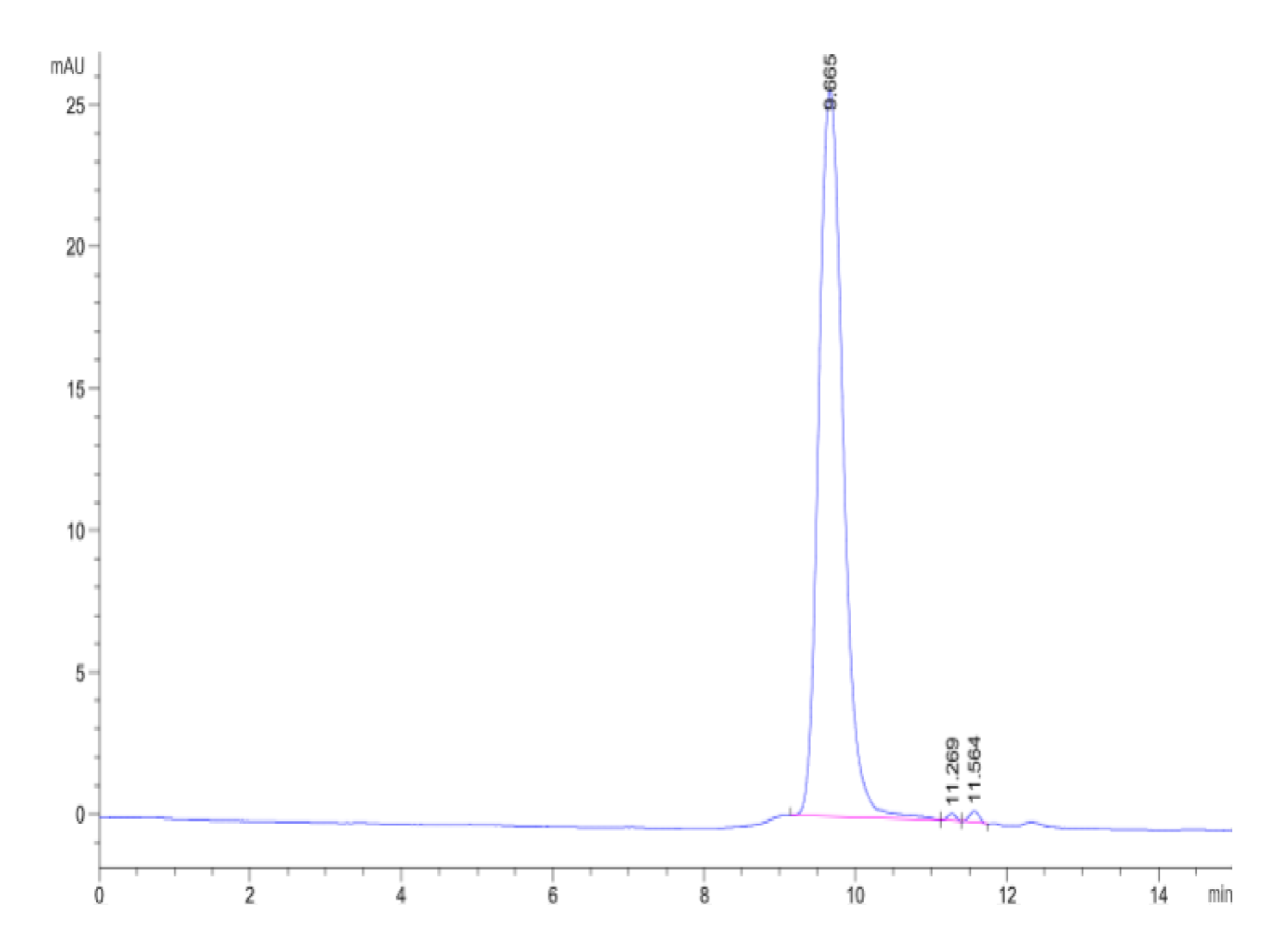
- Purity: > 95% as determined by Bis-Tris PAGE;> 95% as determined by HPLC
- Activity: Immobilized Biotinylated Human DLL3 Domain (311-479), His Tag at 0.5ug/ml (100ul/well) on the streptavidin precoated plate (5ug/ml). Dose response curve for Anti-DLL3 Antibody, hFc Tag with the EC50 of 2.4ng/ml determined by ELISA.
- Amino Acid Range: Val311-Ala479
-
Other Available Proteins:
Loading...
Biotinylated Human DLL3 Domain (311-479) Protein (DLL-HM4D1B) Summary
Product Description |
Recombinant Biotinylated Human DLL3 Domain (311-479) Protein is expressed from HEK293 with with His tag and Avi tag at the C-Terminus.It contains Val311-Ala479. |
Product Background |
Delta-like protein 3 (DLL3) is a transmembrane protein that belongs to the Delta/Serrate/Lag-2 (DSL) family of Notch ligands. DLL3 inhibits primary neurogenesis. May be required to divert neurons along a specific differentiation pathway. Plays a role in the formation of somite boundaries during segmentation of the paraxial mesoderm (By similarity). |
Product Category |
Recombinant Protein / Antibody discovery / ADC targets |
Biotinylated Human DLL3 Domain (311-479) Protein (DLL-HM4D1B) Specifications/Details
Protein |
DLL3 |
Synonyms |
Delta3; DLL3; Pudgy; SCDO1; SCDO1delta3 |
Accession |
|
Species |
Human |
Biotinylated |
yes |
Amino Acid Range |
Val311-Ala479 |
Molecular Weight |
The protein has a predicted MW of 21.20 kDa. Due to glycosylation, the protein migrates to 25-30 kDa based on Bis-Tris PAGE result. |
Activity |
Immobilized Biotinylated Human DLL3 Domain (311-479), His Tag at 0.5ug/ml (100ul/well) on the streptavidin precoated plate (5ug/ml). Dose response curve for Anti-DLL3 Antibody, hFc Tag with the EC50 of 2.4ng/ml determined by ELISA. |
Product Tag |
C-His-Avi |
Fluorophore |
Biotin-labeled |
Expression System |
HEK293 |
Purity |
> 95% as determined by Bis-Tris PAGE;> 95% as determined by HPLC |
Endotoxin |
Less than 1 EU per ug by the LAL method. |
Shipping, Reconstitution, & Storage
Form |
Lyophilized |
Shipping |
Shipped at ambient temperature. |
Formulation |
Lyophilized from 0.22 um filtered solution in PBS (pH 7.4). Normally 8% trehalose is added as protectant before lyophilization. |
Reconstitution |
Dissolve the lyophilized protein in distilled water. It is recommended to resuspend at 0.5 mg/mL if the lyophilized powder is 100 ug or less, at 1mg/ml for 500ug or 1mg lyophilized powder. Do not mix by vortex or vigorous shaking. |
Stability And Storage |
-20 to -80°C for 12 months as supplied from date of receipt.;-80°C for 3 months after reconstitution.;Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
Validation Images
Related Products
Have more questions?
Check out our Frequently Asked Questions page for more details about product specifications, ordering, and shipments.
Contact Us
Recommended Products
Recently viewed