Engineered T7 RNA Polymerase to Reduce dsRNA
By Mallory Griffin
Our Chief Technology Officer, ManHee Suh, recently presented at the NextGen RNA Summit in Boston, MA his talk on "Engineered T7 RNAP for decreased production of dsRNA". Our protein engineering team has developed various mutants of T7 RNA Polymerase (T7RNAP) that produce less immunostimulatory dsRNA during in vitro transcription than wild type and commercial T7RNAP. Additionally, we have developed assay kits for analysis of transcripts including dsRNA, 3'-extensions, circRNA, processivity, and yield. Find a preview of the results of our in-house Acridine Orange dsRNA Assay data below or submit the form below to receive information on test samples and to get a full copy of the presentation and poster.
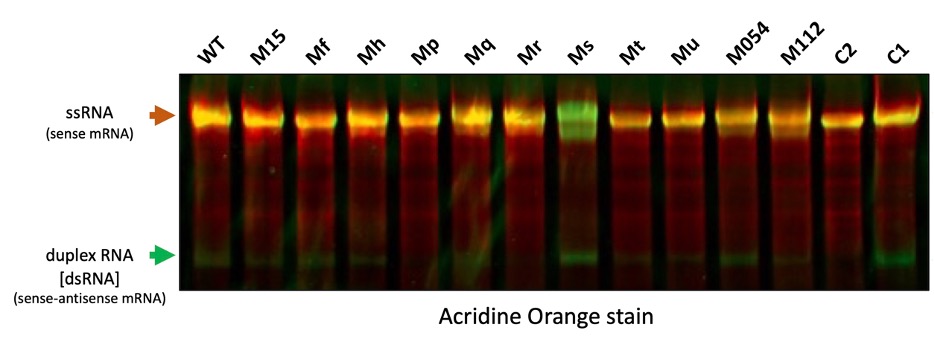
Figure 1. dsRNA byproducts analyzed on RNAs transcribed using our T7RNAP mutants via acridine orange. Acridine orange intercalates into dsRNA and produces green fluorescence at 530 nm and electrostatically binds to the phosphate groups of single-stranded mRNA and produces red fluorescence at 640 nm.
Abstract
In order for in vitro synthesis to be used for therapeutic purposes, it must be reliable and effective, not only for economic but also for safety reasons. Even though in vitro transcription (IVT) utilizing T7 RNA polymerase (T7 RNAP) for the synthesis of messenger RNA (mRNA) is efficient and well-established for the production of large quantities of synthetic RNA, T7 RNAP can generate undesirable, immunostimulatory mRNA byproducts such as double-stranded RNA (dsRNA). Several strategies, including post-synthesis purification methods and optimization of reaction constituents, have been proposed to reduce such byproducts. Purification techniques require the use of special resins and complex purification processes, while the optimization of reaction constituents necessitates customized conditions but is not 100% effective. To reduce these concerns, we rationally engineered a series of T7 RNAP mutants and compared their functional properties to those of wild-type and commercially available T7 RNAP. Among these characteristics are dsRNA, circular RNA, processivity, and yield. We demonstrate that a few engineered variants have the potential to be used in a simplified production process with less immunostimulatory content and less complicated manufacturing. In the present work, the screening of optimized T7 RNAP variants is described.