For commercial-scale mRNA manufacturing.
High Yield
Batch-to-Batch Consistency
Low dsRNA
Stability Testing
Inventory In Stock

Regulatory Documentation
Manufactured according to GMP standards.
Quality Release Criteria
Activity (Molecular Beacon): ≥ 50 kU/mL
Purity (SEC-HPLC): ≥ 95%
Residual Endonuclease: Negative
Residual Exonuclease: Negative
Residual DNase: Negative
Residual RNase: Negative
Residual Protease: Negative
Endotoxin: ≤ 10 EU/mL
Residual Host Cell DNA: ≤ 100 pg/mL
Residual Host Protein: ≤ 20 ng/mg
Residual Heavy Metal: ≤ 10ppm
Bioburden: ≤ 1 CFU/10mL
GMP-Grade Manfuacturing
→ Manufactured in a cGMP facility
→ Digital manufacturing execution system (MES)
10000m2 manufacturing site for GMP production
→ Comprehensive product release process
→ Process control and optimization procedures
→ Comprehensive suite of analytical equipment
→ Stability testing
→ Free from animal-derived materials

Customizable GMP Documentation Package
→ Datasheet
→ CoA
→ CoO
→ MSDS
→ Melamine Statement
→ TSE/BSE Statement
→ Nitrosamine Statement
→ DMF Filing
About T7 RNA Polymerase
T7 RNA Polymerase (T7RNAP) is a protein encoded by phage T7 DNA recombinantly expressed in Escherichia coli. T7RNAP, a DNA-dependent 5'→3' RNA polymerase, highly specifically recognizes the T7 promoter sequence (5'-TAATACGACTCACTATAG-3'). The template is single-stranded or double-stranded DNA containing the T7 promoter sequence. T7RNAP synthesizes an RNA strand using NTPs, complementary to the DNA template, downstream of the promoter.
About Our Enzyme Engineering & Expression Optimization
We engineered our T7 RNA Polymerase using Structure Aided Multiplex Screening (SAMS), our unique innovative functional recombinant protein engineering platform. It has also undergone optimization of the E. coli expression system, purification process, and buffer formulation. We've designed our T7 to produce low error rates, high efficiency, and high processivity. We've optimized the enzyme for yield and purity, making it both practical and affordable for large-scale production of mRNA / mRNA synthesis.
About Our GMP Compliance
We manufacture our T7 RNA Polymerase in our cGMP compliant facility. Moreover, we've submitted our GMP T7RNAP to the FDA Drug Master Files (DMF #037660). Customizable regulatory support documentation including batch production records, stability testing, CoA, etc. is available.
Customizable GMP Documentation Package:
→ Datasheet
→ CoA
→ CoO
→ MSDS
→ Melamine Statement
→ TSE/BSE Statement
→ Nitrosamine Statement
→ DMF Filing
Concentration: 50U/µL
Unit Definition: One unit is defined as the amount of enzyme required to incorporate 1 nmol ATP into acid-insoluble material in a total reaction volume of 50µL in 1 hour at 37°C.
Source: Expressed in an E.coli strain that carries the T7 RNA Polymerase gene.
→ Manufactured according to GMP guidelines
→ FDA Drug Master Files #037660
→ Antibiotic-free and animal-free production
Product Information
Quality Release Specifications

ISO13485:2016 certification
ISO13485:2016 certification is an internationally recognized standard that sets out the requirements for a quality management system that ensures companies adhere to stringent quality control measures, regulatory compliance, and risk management practices throughout the entire product lifecycle. Our company has obtained ISO13485:2016 certification to demonstrate our commitment to producing safe, reliable, and high-quality proteins and enzymes.
Approval ID: 00034327

FDA Drug Master Files #037660
A DMF (Drug Master File) is a document submitted by a company to the FDA. The document contains detailed information on the entire production process, including product formulations, plant facilities, production processes, and any other procedures involved in this product. DMF filing demonstrates transparency by the company and willingness to work alongside regulations. Additionally, when companies apply for a new product registration with the FDA, using materials with a DMF will shorten the registration time.
Product Performance Validation
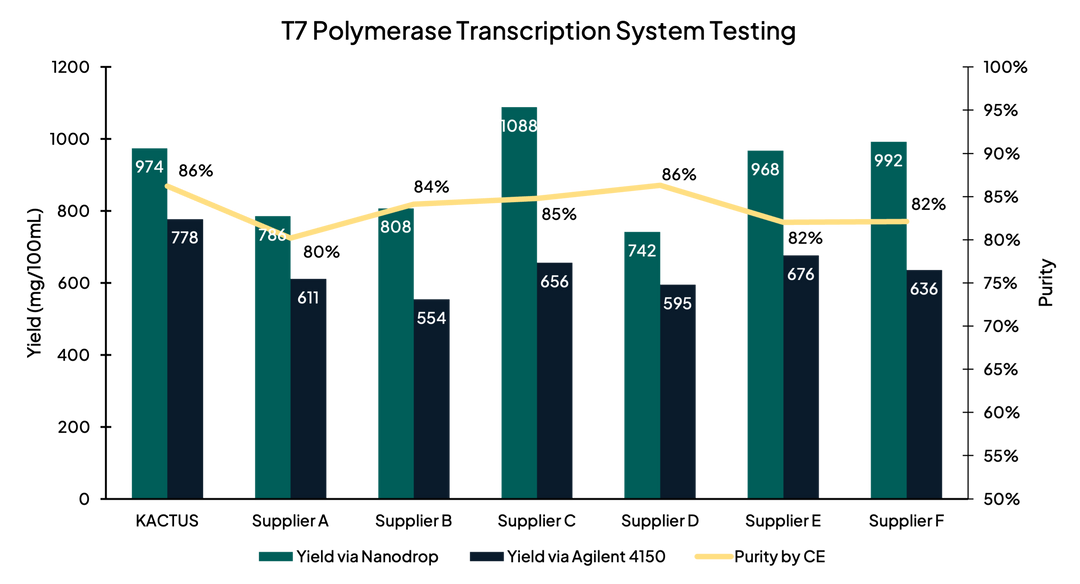
Overall Performance
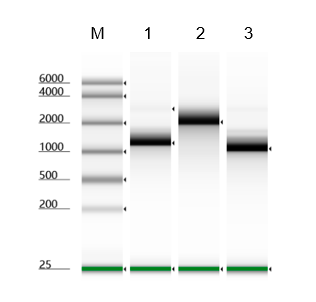
Figure 1. After synthesizing mRNA in an IVT reaction, we assessed RNA transcript yield using nanodrop and Agilent 4150 tapestation. We assessed purity using capillary electrophoresis (CE). Overall, yield and purity of our T7RNAP was comparable to leading suppliers.
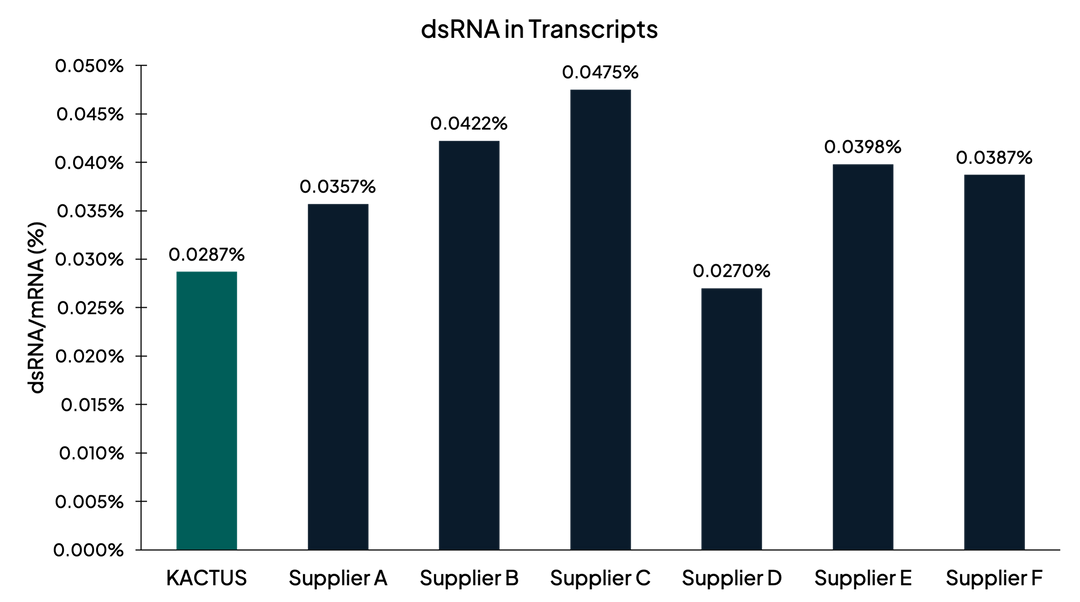
Low dsRNA in Transcripts
Figure 2. dsRNA measured after in vitro transcription using J2-based ELISA. KACTUS T7 has low dsRNA contamination comparable or superior to leading competitors.
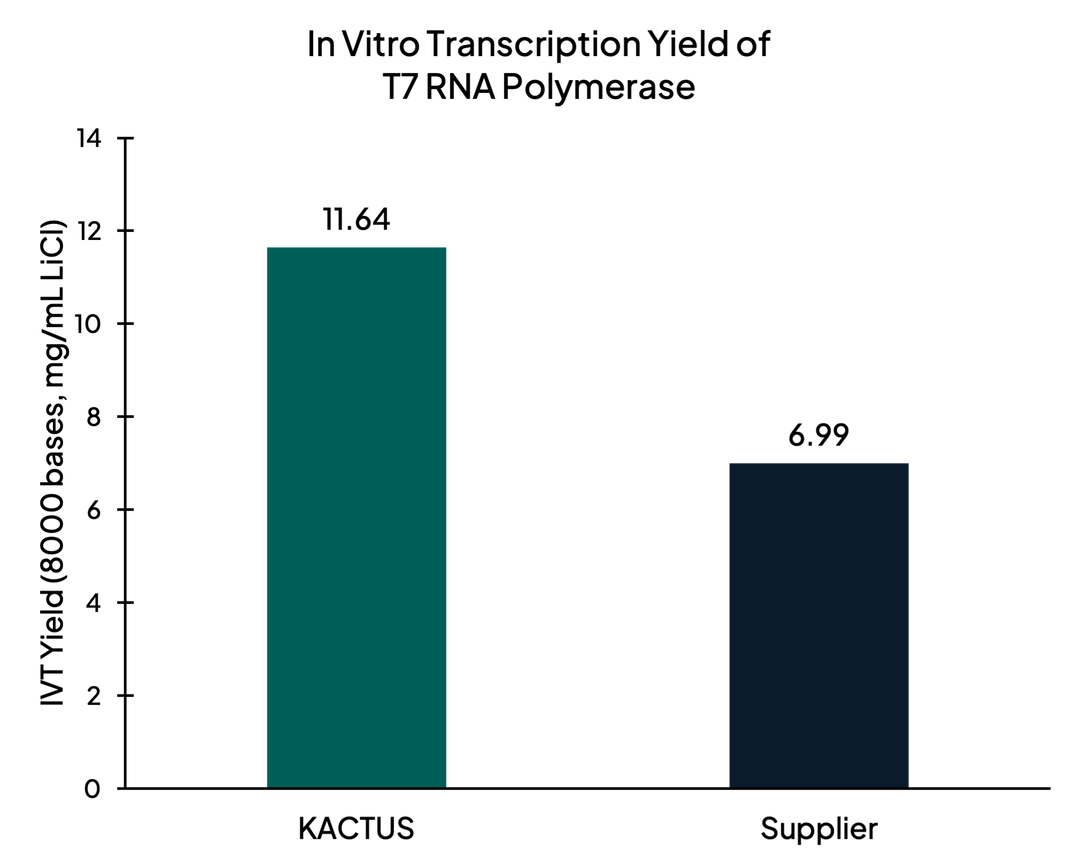
High Transcript Yield
Figure 3. After synthesis of mRNA, in vitro transcription yield of T7 RNA Polymerase measured with Agilent 4150 Tapestation. KACTUS T7 had 65% higher yield than leading supplier.
Batch Consistency
Figure 4. Detection of T7 RNA Polymerase by molecular beacon. We analyzed three batches of T7 RNA Polymerase and found similar reaction kinetics across all batches.
Ordering Information
Request a Sample
Contact us at sales@kactusbio.us to inquire about a sample of our GMP T7 RNA Polymerase. Alternatively, submit the sample request form below.
Product Page
To view product information or place an order online, please visit the GMP-Grade T7 RNA Polymerase product page. To order via PO, you may send your order information directly to orders@kactusbio.us.
Bulk Quote
Our T7 RNA Polymerase is available in routine bulk supply for large-scale mRNA manufacturing. To request a bulk quote, please contact sales@kactusbio.us or click below to submit a bulk quote request.
Related Products
Get in Touch
Would you like more information regarding our GMP T7 RNA Polymerase? Contact us today to request more information regarding our T7 RNA Polymerase or set up a quote. Our team is eager to discuss your specific manufacturing needs.
GMP Grade T7 RNA Polymerase FAQs:
T7 RNA Polymerase is used in in vitro transcription (IVT) to generate RNA from DNA templates containing a T7 promoter. It supports applications such as mRNA synthesis, RNA probe generation, hybridization studies, and RNase protection assays.
This enzyme recognizes and binds specifically to the T7 promoter sequence (5'-TAATACGACTCACTATAG-3'). Transcription begins immediately downstream, ensuring targeted and efficient RNA synthesis.
GMP-grade enzymes comply with strict regulatory and quality control standards. They provide high purity and reproducibility, making them suitable for clinical RNA production and regulatory submissions, including DMF referencing.
GMP T7 RNA Polymerase typically exhibits ≥95% purity (SEC-HPLC) and ≥200 kU/mL activity measured by molecular beacon assays. It also meets criteria for endotoxin levels, host cell DNA/protein, and enzyme residue limits.
Optimized transcription conditions and proprietary buffer systems are used to minimize dsRNA byproducts. Each batch is validated using J2 antibody-based ELISA, confirming suitability for clinical-grade mRNA production.
Each batch undergoes molecular beacon-based activity testing and kinetic comparison to verify uniformity across production lots. This supports consistent transcript yield and purity.
A comprehensive documentation package is available, including the Certificate of Analysis (CoA), Certificate of Origin (CoO), MSDS, TSE/BSE and Melamine statements, and FDA Drug Master File (DMF) support documents.
Yes. The enzyme is designed for commercial-scale use, with high concentration formats and validated lot consistency to meet bulk production demands.
The enzyme should be stored at –20 ± 5°C and should not be subjected to repeated freeze-thaw cycles to preserve activity.
T7 RNA Polymerase is recombinantly expressed in E. coli and purified through multiple chromatography steps to remove RNases, DNases, proteases, and other contaminants. The result is a highly pure and functionally consistent enzyme suitable for sensitive applications.