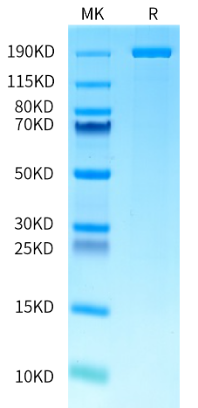
NK Cell Therapy Gets IND FDA Clearance with AccuBase® Base Editor from KACTUS
By Mallory Griffin
November 14, 2024 Base Therapeutics announced that its independently developed NK510 cell injection, the world's first base-edited universal NK cell product, has received IND clearance from the U.S. Food and Drug Administration (FDA). The NK510 Cell Injection is intended for the treatment of advanced-stage solid tumors. KACTUS is proud to be the exclusive supplier of AccuBase® cytosine base editor, the critical raw material for the clinical manufacturing of NK510 cell injection, as well as providing regulatory support for the IND application.
NK510 is an off-the-shelf universal NK cell therapy independently developed by Base Therapeutics. It leverages AccuBase®, a low-off-target cytosine base editor, to edit key genes in NK cells, further enhancing NK510's ability to accurately evaluate the activation and inhibition signals within the tumor microenvironment, and achieving a dual effect of overcoming cancer cell immune evasion while specifically recognizing and killing cancer cells. Currently, NK510 has demonstrated strong anti-solid tumor capabilities in multiple in vitro and in vivo preclinical studies.

Figure 1. Advantages of Base Therapeutics NK Cell Therapies [1].
As part of their cell therapy efforts, Base Therapeutics has successfully developed a robust process for NK cell expansion. This process enables million-fold cell expansion and stable multi-batch production. This process successfully overcomes low expansion rates and low transfection efficiency, the global challenges of allogeneic NK cell production.
About AccuBase® Cytosine Base Editor
NK510 uses AccuBase® cytosine base editor to produce genetically engineered NK cells. AccuBase® is the world’s first commercially available base editor, developed by Base Therapeutics and manufactured and distributed exclusively by KACTUS. Its high-efficiency and precise base editing capabilities have been validated across multiple cell types, achieving near-zero off-target effects. Currently, AccuBase® is available globally in both research-grade and GMP-grade, supporting the development of preclinical and clinical gene editing therapies. AccuBase® has received freedom-to-operate (FTO) designation globally.
AccuBase® Base Editor Mechanism of Action
After forming a ribonucleoprotein (RNP) with sgRNA, AccuBase® remains in a non-editing state until it binds to the target DNA. The deaminase domain is encapsulated within the Cas9n protein, preventing interaction with non-target DNA and significantly reducing off-target effects. Upon binding to the target DNA, AccuBase® undergoes a conformational change, exposing the deaminase domain to precisely edit bases within the 3-12 window range of the target site (Figure 2). This targeted editing allows for specific point mutations (C to T or G to A) or gene knockouts by introducing premature stop codons or altering splicing sites, such as disrupting splice acceptor (SA) or splice donor (SD) sites, making it ideally suited for single-point mutation corrections or gene knockouts.

Figure 2. Schematic of AccuBase® base editing mechanism.
Advantages of AccuBase® Base Editor
- No Double-Stranded Breaks AccuBase® directly modifies target bases without causing double-stranded DNA breaks, unlike traditional CRISPR-Cas9/Cas12 nucleases. This avoids unexpected mutations causing genomic instability, chromosomal rearrangements, and p53 inactivation.
- Near-Zero Off-Target Effects The unique structural design of AccuBase® minimizes the occurrence of non-specific mutations, significantly enhancing the safety, reliability, and consistency of the editing process.
- High Editing Efficiency AccuBase® achieves an average editing efficiency of over 90% across randomly selected genomic loci.
- Broad Applicability The activity of AccuBase® has been validated on hundreds of gene loci in multiple cell lines such as human T cells, NK cells, and ESC stem cells, as well as other mammalian and fish cell lines.
- First Protein-Based Base Editor Nowadays, transient RNP (ribonucleoprotein) delivery has become the preferred delivery method for ex vivo gene editing. KACTUS has optimized the production process for AccuBase® enzyme, ensuring exceptional stability. Both research-grade and GMP-grade AccuBase® proteins are now available to meet quality and compliance needs at different pipeline stages.
- Global Patent Protection AccuBase® has obtained multiple invention patents in China, Europe, and the U.S. and has received full global freedom-to-operate (FTO) designation.
- International Recognition and Open Collaboration Base Therapeutics has granted non-exclusive licenses for AccuBase® base editing technology to leading CAR-T therapy companies.
Performance Validation of AccuBase® Base Editor
High Base Editing Activity
Figure 3. AccuBase® RNP was electroporated into activated primary T cells. According to flow cytometry, AccuBase® can efficiently knock out PD1, B2M, and TRAC proteins on the membrane of activated primary T cells at the protein level. For PD1 and B2M, the knockout efficiency exceeded 80%, while the knockout efficiency of TRAC reached 96%. FACS efficacy % = (positive control - KO group)/positive control* 100%.
Near-Zero Off-Target Levels

No DNA Insertions, Deletions, or Chromosomal Translocations

Figure 5. Compared with Cas9 protein, AccuBase® does not produce DNA horizontal insertions and deletions (INDELs).
Figure 6. Compared with Cas9 protein, AccuBase® does not cause chromosomal translocation.
AccuBase® Product List
|
Catalog No. |
Product Description |
Available Sizes |
|
1mg |
||
|
100μg / 500μg / 1mg |
||
|
96 Tests |
Note: AccuBase® is the commercial name for BS-EP1.
About Base Therapeutics
Base Therapeutics was founded in April 2021, specializing in the research and development of novel gene-editing NK cell therapy and gene therapy products. The company operates a 2,500-square-meter GMP cell manufacturing facility and a 1,500-square-meter laboratory to meet the rigorous demands of clinical-grade cell therapy R&D and production. Base Therapeutics is conducting clinical research on its base-edited NK cell therapies to treat advanced solid and hematological tumors, including osteosarcoma, soft tissue sarcoma, non-small cell lung cancer, and acute myeloid leukemia. In addition to NK510, Base Therapeutics pipeline includes base-edited CAR-T products and in vivo base editing therapies.
About KACTUS
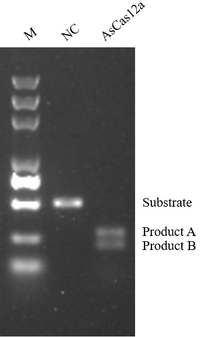
KACTUS is an innovation-driven biotech specializing in recombinant proteins and enzymes for novel therapeutic development. Since its establishment in 2018, KACTUS has accumulated comprehensive experience in protein design and expression by producing high-quality engineered enzymes and complex protein targets. KACTUS operates a 10,000 m² GMP production facility and certified quality management system, manufacturing various GMP-grade proteins and enzymes for cell and gene therapy drug development. In addition to AccuBase®, KACTUS offers an off-the-shelf GMP-grade Cas9 protein and GMP-ready Cas12a protein, further supporting cell and gene therapy applications with high-quality, regulatory-compliant raw materials.
Access More Information
For more information on AccuBase® base editor, access our brochure or visit the product page. To learn more about our gene editing product offerings, browse our Gene Editing Enzymes & Reagents.
References
- https://www.basetherapeutics.com/index.html#12