Exploring RNP: A Cutting-Edge Tool for Gene Delivery
By Yujiao Zhang
Through decades of rapid development, gene editing technology has officially entered the CRISPR 2.0 era, with new technologies such as base editing, prime editing, and epigenomic editing emerging from academia to industry. Meanwhile, a plethora of novel CRISPR technologies discovered with the aid of AI have significantly expanded the gene editing toolkits. Biotech companies are actively strategizing and demonstrating strong innovative capabilities, such as HuidaGene, the developer of hfCas12Max®, and Base Therapeutics, who developed AccuBaseTM, the world's first-on-market cytosine base editor.
CRISPR technology enables precise modification of the genome, exhibiting splendid potential for cell and gene therapy development targeting rare or hereditary diseases. In clinical applications, the usage of Ribonucleoprotein (RNP) is rapidly emerging and highly favored in the current delivery method development for gene therapy.

Table 1. Clinical applications of RNP-mediated genome editing [1].
Advantages of RNP
Broad applicability
- Applicable to multiple cell types: The RNP form of CRISPR/Cas editing tools can be effectively delivered to a variety of cell types, including cells that are typically difficult to transfect, such as immune cells and stem cells. It has been widely applied in the clinical trials.
- Applicable to cells from various species: The RNP delivery method can also be used in species other than mammals without consideration for codon preference.
High gene editing efficiency
- By directly delivering the RNP into cells, the enzymes and gRNAs can function immediately without undergoing transcription and translation, achieving maximum editing efficiency within 24 hours [2].
High Safety
- Rapid editing and clearance cycle: RNP can be rapidly degraded after the gene editing is completed, with almost no detectable Cas protein after 24-48 hours [3]. Such a short half-life minimizes the possibility of off-target effects, ensuring the safety of gene editing for patients.
- Reduced risk of the immune response: Due to the transient presence and rapid degradation of RNP, it lowers the risk of triggering immune responses.
- Adjustability: The RNP delivery system can fine-tune the amount of sgRNAs and Cas proteins in vitro to meet specific needs under different scenarios.
Easy to use
- Compared to traditional gene editing methods, the delivery process of RNP is faster and more straightforward, making it a cost-saving and time-saving option for researchers.
RNP Delivery
Unlike pure proteins or nucleic acids, the complex composition and charge characteristics of RNP make it a challenge to effectively deliver it into cells. So far, there are three approaches to delivering RNP into cells: 1) Physical approach, such as electroporation or microinjection; 2) Nanoparticles, such as LNPs; and 3) virus-like particles. Clinically, electroporation is most frequently used to deliver RNP to primary cells [4].

Figure 1. Methods of RNP delivery [1].
Challenges and Prospects of RNP
Off-target risk
Although the RNP features certain advantages in reducing off-target risks, the chance of occurrence cannot be completely mitigated. Fortunately, the MHRA (UK) and FDA (US) have recently approved Casgevy, the RNP-based CRISPR/Cas therapy collaboratively developed by Vertex and CRISPR Therapeutics [5]. This is the world's first approved CRISPR gene editing therapy and therefore, sets the foundation for further development of CRISPR-based therapies.
in vivo delivery of RNP
In addition to the activity of RNP and efficient cell entry, in vivo gene editing also requires precise targeting of organs or tissues. Hopewell Therapeutics, established in 2018, has successfully developed organ-specific LNP technology that can deliver various forms of gene editing complexes including RNP; Nvelop Therapeutics, founded by David Liu, also developed engineered virus-like particles (eVLP) with promising potential for RNP delivery in vivo [6].
Manufacturing
The current wild-type Cas9 proteins and certain Cas9 variants on the market are no longer sufficient to meet the increasingly stringent standards of targeting as required by drug development and clinical applications. To meet these high demands, more Cas or Cas-related fusion proteins with low off-target rates and high editing efficiency are being developed. Yet, these proteins are still difficult to obtain as off-the-shelf products, especially fusion proteins (such as Base Editor BE3) with larger molecular weights, posing great challenges for in vitro expression and purification [7]. Developing a process to increase the yield and purity while still maintaining the enzyme activity to meet GMP Grade or CGT standards is a challenging problem. This urgent issue can often be addressed by custom recombinant protein expression services, which enable tailored production strategies to optimize complex proteins like gene editors and fusion enzymes.
The ‘In-stock’ Atlas of Gene Editing Enzymes from KACTUS
KACTUS currently provides the following research-grade and GMP-grade gene editing enzymes for research. Through continuous refinement of its technology platform, KACTUS will offer more and more reliable gene editing tools, thereby contributing to the advancement and widespread application of gene editing technology for therapeutic purposes.
→ Cas9
GMP-grade, FDA DMF (Drug Master File) registered, successfully applied in IND (Investigational New Drug) clinical pipelines.
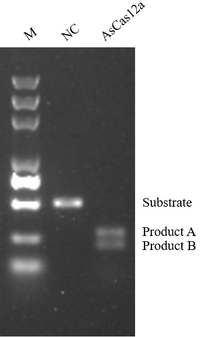
→ AsCas12a
High gene editing efficiency, staggered (sticky ends) cut, smaller RNA molecule size, providing more options to fulfill different needs.
→ AccuBaseTM
GMP-grade Cytosine Base Editor (CBE), does not generate DNA double-strand breaks, nearly zero off-target risk; independently developed by Base Therapeutics and authorized to KACTUS the rights of global manufacturing and commercialization.
|
Catalog |
Name |
Size |
|
Cas9 Nuclease (GMP-Grade) |
3mg |
|
|
Cas9 Nuclease (Research-Grade) |
100μg/1mg |
|
| CAS-MM00B | Cas9 (CRISPR Associated Protein 9) ELISA Kit | 96T |
|
SpCas9 D10A Nickase (GMP-Ready) |
100ug/1mg |
|
|
AsCas12a Nuclease (GMP-Ready) |
100ug/1mg |
|
|
BS-EP1 (AccuBaseTM GMP-Grade) |
1mg |
|
|
BS-EP1 (AccuBaseTM Research-Grade) |
100μg/200ug/500ug/1mg |
Click catalog number for product details.
Note: BS-EP1 is product name, AccuBaseTM is commercial name
References
[1]. Bloomer H, Khirallah J, Li Y, Xu Q. CRISPR/Cas9 ribonucleoprotein-mediated genome and epigenome editing in mammalian cells. Adv Drug Deliv Rev. 2022 Feb;181:114087. doi: 10.1016/j.addr.2021.114087.
[2]. Kim S, Kim D, Cho SW, Kim J, Kim JS. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014 Jun;24(6):1012-9. doi: 10.1101/gr.171322.113.
[3]. Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, Carte J, Chen W, Roark N, Ranganathan S, Ravinder N, Chesnut JD. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol. 2015 Aug 20;208:44-53. doi: 10.1016/j.jbiotec.2015.04.024.
[4]. Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020 Feb;578(7794):229-236. doi: 10.1038/s41586-020-1978-5.
[5]. FDA News Release on Dec 08, 2023: FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease.
[6]. Raguram A, Banskota S, Liu DR. Therapeutic in vivo delivery of gene editing agents. Cell. 2022 Jul 21;185(15):2806-2827. doi: 10.1016/j.cell.2022.03.045.
[7]. Rees HA, Minella AC, Burnett CA, Komor AC, Gaudelli NM. CRISPR-derived genome editing therapies: Progress from bench to bedside. Mol Ther. 2021 Nov 3;29(11):3125-3139. doi: 10.1016/j.ymthe.2021.09.027.