Recombinant GPCR Proteins
Recombinant GPCR Proteins
G-protein coupled receptors, also known as GPCRs, are the largest and most diverse group of cell surface receptors in eukaryotic cells. As integral membrane proteins, they have 7 transmembrane helices, a feature necessary for their function.
GPCRs are involved in many physiological processes, from vision, taste, and smell to mood regulation and immune response. This also extends to smooth muscle contraction and systems throughout the body, including the cardiovascular and digestive tracts. Since they are central to cellular communication, GPCRs are a prime target for drug discovery.
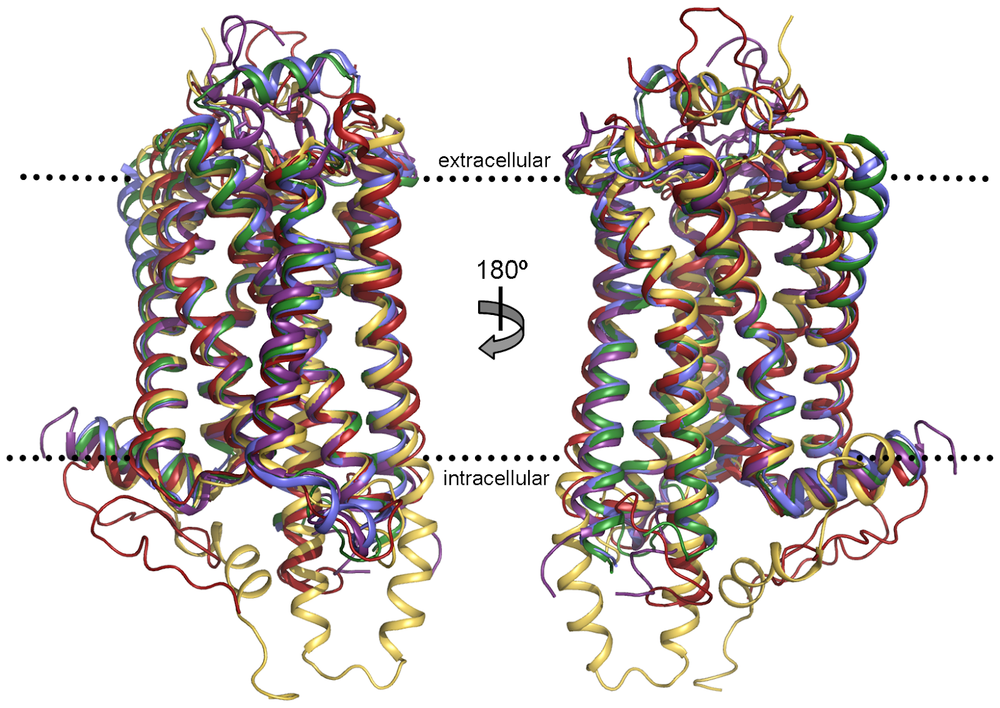
GPCRs have 7 transmembrane helices connected by intracellular and extracellular loops. The N-terminus of the receptor is on the extracellular side of the membrane, and the C-terminus is on the intracellular side. The ligand binding site of the receptor is typically within the transmembrane helices.
When ligands bind to GPCRs, they change conformation and activate nearby heterotrimeric G proteins in the cytoplasm. G proteins are complex molecules composed of three subunits: alpha, beta, and gamma. The alpha and gamma subunits are anchored to the plasma membrane by lipids. In their inactive state, the G protein alpha subunit binds GDP and is associated with the beta-gamma subunits and the GPCR [2].
The conformational change of GPCR causes the alpha subunit to release GDP and bind GTP instead, triggering the dissociation of the alpha subunit from the beta-gamma dimer. While remaining anchored to the membrane, both subunits are free to move laterally and interact with other membrane proteins, such as enzymes that generate second messengers or ion channels.
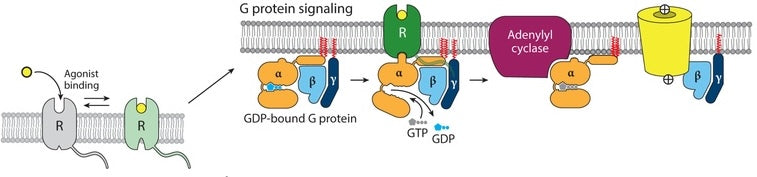
Figure 2. Mechanism of GPCR activation upon agonist binding: Agonist attachment to receptor (R) initiates the classical G protein pathway, prompting the G protein α subunit to exchange GDP for GTP. This exchange triggers the α subunit’s dissociation and subsequent interaction with downstream targets, leading to the stimulation of adenylyl cyclase by Gαs and the activation of ion channels by Gβγ [3].
These interactions amplify the signal, different G proteins either stimulating or inhibiting their targets and leading to many different cellular responses. This is a temporary signal, the alpha subunit will eventually hydrolyze the bound GTP back to GDP and reassociate with the beta-gamma subunits and the inactive GPCR, resetting the protein for another signal cycle.
This illustrates how GPCRs act as molecular switches, converting external chemical messages into internal cellular responses. This makes them a prime target for drug development for many diseases.
The human genome encodes for over 800 different G-protein coupled receptors (GPCRs), the largest family of signaling receptors. These receptors are essential for cellular communication and are central to physiological responses. GPCRs are classified into several classes based on their sequence homology and functional characteristics. The main classes are Class A (rhodopsin-like), Class B (secretin-like), Class C (glutamate receptor-like), and other miscellaneous classes.
Class A GPCRs are the most abundant and include receptors for sensory perception, such as light and odorants.
Class B includes receptors for hormones such as glucagon and calcitonin, which are involved in metabolic regulation.
Class C GPCRs are involved in neurotransmission and include the metabotropic glutamate receptors.
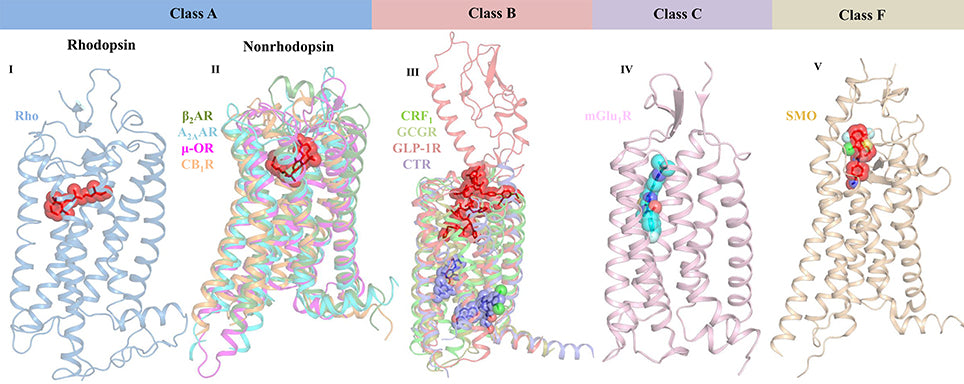
Figure 3. Crystal structures reveal the varied ligand-binding sites of representative GPCRs from classes A, B, C, and F.
The diversity of GPCRs is behind their involvement in many physiological processes, including vision, taste, olfaction, and behavioral response to environmental stimuli. This makes them a key target in pharmacological research and drug development. This classification system not only gives insight into receptor function but also helps in designing new therapeutic agents.
GPCRs activate many different signaling pathways depending on the receptor and the G protein involved. Some of the major GPCR signaling pathways:
cAMP pathway: This pathway is activated by Gs proteins and generates cyclic AMP (cAMP), a second messenger that activates protein kinase A (PKA). PKA then phosphorylates downstream targets and leads to a cellular response.
Phosphatidylinositol pathway: This pathway is activated by Gq proteins and generates inositol trisphosphate (IP3) and diacylglycerol (DAG), second messengers that activate protein kinase C (PKC) and release calcium from internal stores respectively.
MAPK pathway: This pathway is activated by various G proteins and leads to the activation of mitogen-activated protein kinases (MAPKs), which are involved in cell growth, differentiation, and apoptosis.
cAMP signaling pathway: This pathway is activated by Gs proteins and leads to the production of cyclic AMP (cAMP), a second messenger that activates protein kinase A (PKA). PKA then phosphorylates a variety of downstream targets, leading to a cellular response.
GPCR signaling is complex, involving multiple steps of activation, signaling through G-proteins, and eventual regulation of downstream effectors such as enzymes, ion channels, and other cellular components. The classical view of GPCR signaling is the G-protein pathway, but β-arrestins also mediate some GPCR signals, which can influence cell behavior independently of G-proteins [5].
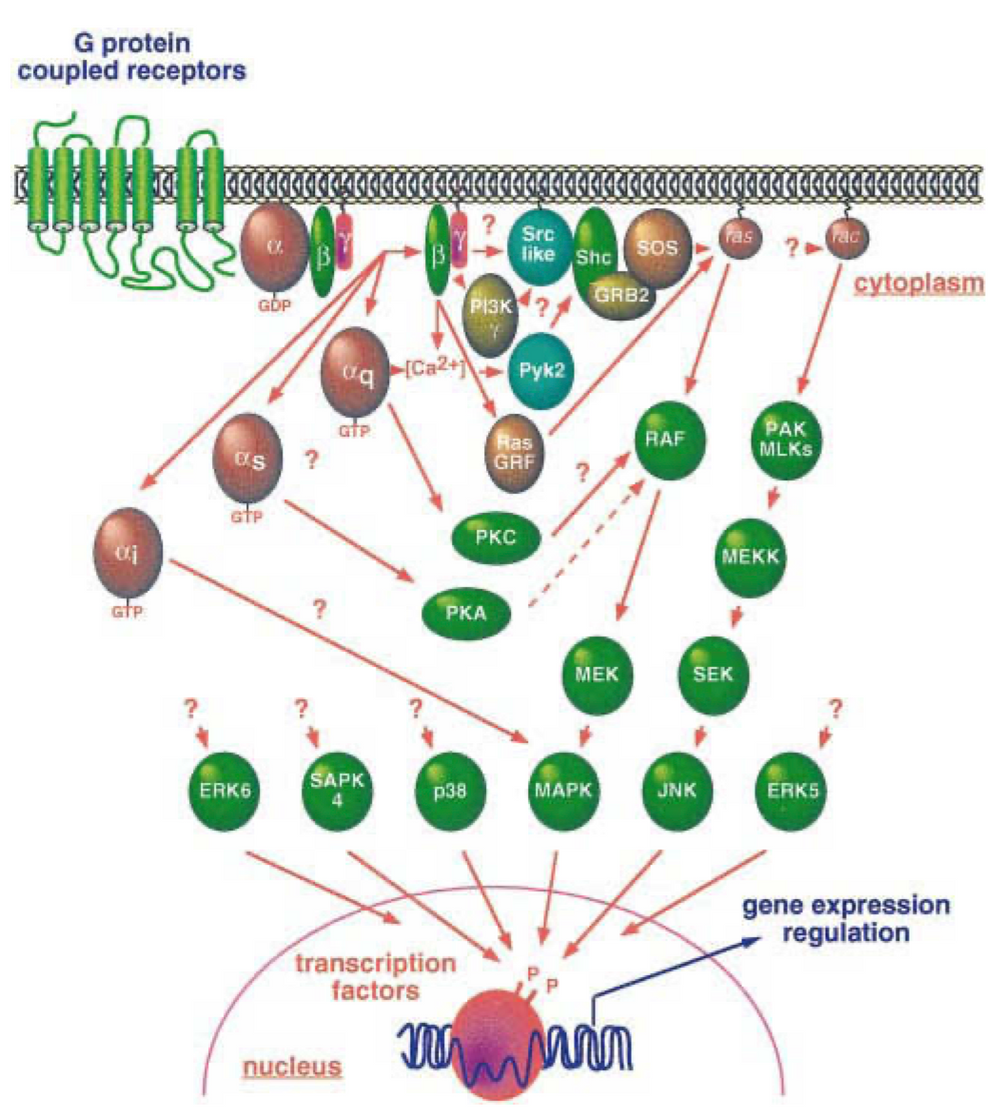
Figure 4. GPCR signaling activates diverse MAPK cascades, influencing nuclear function. While the pathways connecting GPCRs to small GTPases and other MAP kinase family members are still being investigated, these cascades ultimately target key cellular processes [4].
Since they are on the cell surface and central to many signaling pathways, GPCRs are an attractive therapeutic target. About 34% of all FDA-approved drugs target GPCRs. Their dysregulation is implicated in many disease areas, such as cardiovascular, diabetes, cancer, and depression. Some of the diseases associated with GPCR dysfunction include:
GPCRs play a crucial role in cardiovascular health by regulating heart rate, blood pressure, and vascular tone. Dysregulation of GPCR signaling can lead to hypertension, heart failure,e and other cardiovascular disorders. For example, the β-adrenergic receptors are essential for heart rate and contractility. Overstimulation of these receptors can lead to heart failure, whereas their blockade is a common treatment for hypertension. Modulating GPCR activity is a mechanism to treat a range of cardiovascular conditions, a major area of focus for drug development.
Recent studies have shown GPCRs as key players in neurodegenerative diseases like Alzheimer’s, Parkinson’s, and Huntington’s disease. These receptors are involved in various neuronal functions, including neurotransmission, synaptic plasticity, and neuroprotection. In neurodegenerative conditions, GPCR dysfunction can contribute to neuronal damage and death. For example, glutamate receptors, a class of GPCRs, are involved in excitotoxicity, a process where overstimulation of neurons leads to their demise. Other GPCRs are involved in protein aggregation, a hallmark of diseases like Alzheimer’s and Parkinson’s. Targeting specific GPCRs will provide therapeutic strategies for these debilitating conditions either by protecting neurons from damage or by modulating the underlying disease processes.
GPCRs regulate glucose homeostasis and insulin secretion. The glucagon-like peptide-1 receptor (GLP-1R) is a GPCR that, when activated, increases insulin secretion and lowers blood glucose levels. Agonists targeting GLP-1R are currently used to treat type 2 diabetes. Understanding and manipulating the signaling pathways of such GPCRs will provide opportunities to develop new diabetic treatments that are more effective and have fewer side effects.
GPCRs also regulate immune system responses and are involved in inflammatory diseases. For example, receptors like histamine receptors are involved in allergic responses, and their blockers are used to treat symptoms of allergies and asthma. GPCRs, like those responding to chemokines, are crucial in the migration of immune cells to inflammation sites. Targeting these receptors can modulate immune responses and provide therapeutic strategies for autoimmune diseases and other inflammatory conditions.
GPCRs are involved in brain function and implicated in depression, anxiety, and schizophrenia. For example, the serotonin receptors, a subgroup of GPCRs, are targeted by many antidepressants. The dysregulation of these receptors can affect neurotransmitter release and uptake, leading to mood imbalances and other symptoms of mental health disorders. Drugs that can selectively target specific GPCRs in the brain will treat these conditions with higher efficacy and potentially fewer side effects compared to broad-spectrum pharmacological agents.
GPCRs are involved in many aspects of tumor development, including cell growth, proliferation, and metastasis. For example, the overexpression of certain GPCRs like CXCR4 and CCR5 is linked to the aggressiveness of breast and prostate cancers. GPCRs can activate downstream signaling pathways that promote cancer cell survival and proliferation. GPCRs are also involved in creating an environment conducive to cancer spread, angiogenesis (formation of new blood vessels), and modifying the immune response against tumor cells. Therefore they are a target for anticancer therapies which aim to block these receptors and halt cancer progression.
Understanding how GPCRs contribute to these conditions will lead to better treatments. For example, the overexpression of certain GPCRs is linked to tumor growth and progression, making them a target for anticancer drugs. The development of biased agonists, allosteric modulators, and other novel ligand types, including antibody drugs, has opened up new ways to target GPCRs with more specificity and fewer side effects.
These are a new class of therapeutics that involve monoclonal antibodies targeting specific GPCRs involved in disease processes. For example, antibodies targeting the chemokine receptors C-X-C chemokine receptor type 4 (CXCR4) and C-C chemokine receptor type 5 (CCR5) are being developed for certain types of cancer and to modulate immune responses in inflammatory diseases. Antibodies against the Adenosine A2A Receptor (A2AR) are being studied for cardiovascular diseases by regulating myocardial oxygen consumption and coronary blood flow. Cannabinoid receptor 1 (CB1) is being investigated for metabolic disorders, pain management, and neuropsychiatric conditions. Unlike small molecule drugs, antibody drugs can achieve high specificity towards their target GPCRs, potentially offering treatments with fewer side effects.
GPCRs are the hub of cellular signaling and, hence, prime drug targets. However, individual variations in GPCR expression—known as GPCR profiles—can significantly impact drug response. Differences in receptor type and abundance can mean a drug that works for one person may not work for another or even cause adverse reactions.
Understanding these individual GPCR profiles is key to personalized medicine. By tailoring treatments to a person’s specific GPCR makeup, we can achieve better targeting of cellular signaling pathways. This means better efficacy and fewer side effects, which is a big leap in personalized medicine. Advances in imaging technologies combined with genomics and proteomics are enabling researchers to create detailed maps of individual GPCR profiles paving the way for truly personalized treatments.
This approach will be particularly useful for diseases where GPCR dysfunction is the underlying cause, such as many neurological disorders, metabolic diseases, and certain cancers. These conditions are often hard to treat with conventional drugs due to the complexity of the affected pathways. Additionally, advances in gene manipulation technologies are opening up new opportunities. The ability to modulate GPCR function precisely offers hope for novel therapies targeting the root cause of these complex diseases, including those with complex genetic and molecular underpinnings.
GPCRs are a large and diverse family of membrane receptors that play a crucial role in cellular responses to external stimuli. There are multiple ways G proteins engage in signal transduction once activated, involving diverse combinations of different subunits. Because they are involved in a range of cellular processes, many FDA-approved drugs target GPCRs.
To facilitate effective GPCR-related therapeutic research, KACTUS offers an off-the-shelf portfolio of recombinant GPCRs with high purity and validated bioactivity. These proteins are displayed on virus-like particles (VLPs) or copolymer nanodiscs to maintain natural protein folding and native conformation of these multi-pass transmembrane proteins. To learn more about our membrane protein display platforms, click here.
These proteins exhibit high binding activity and can be applied to various research stages, including ELISA method development and pharmacokinetic analysis.
Figure 5. Immobilized Biotinylated Human MRGX2, His Tag at 5ug/ml (100ul/well) on the streptavidin precoated plate (5ug/ml). Dose response curve for Anti-MRGX2 Antibody, mFc Tag with the EC50 of 6.2ng/ml determined by ELISA (QC Test).
Figure 6. Immobilized Human A2AR VLP at 5ug/ml (100ul/Well) on the plate. Dose response curve for Anti-A2AR Antibody, mFc Tag with the EC50 of 0.10ug/ml determined by ELISA.
Figure 7. Immobilized Biotinylated Human SSTR2 Nanodisc, His Tag at 2ug/ml(100ul/well) on the streptavidin precoated plate (5ug/ml). Dose response curve for Anti-SSTR2 Antibody, hFc Tag with the EC50 of 6.1ng/ml determined by ELISA (QC Test).
Figure 8. Biotinylated CCR2B VLP captured on CM5 Chip via streptavidin can bind Anti-CCR2 Antibody, hFc with an affinity constant of 2.19 nM as determined in SPR assay (Biacore T200) (QC Test).
The GPCR molecule is the major mechanism regulating our physiological responses to the hormones, neurotransmitters, stimulators, and environmental stimulants, and thus has potential as therapy targets.
G proteins can activate multiple messenger and intracellular responses and induce physiological responses in tissues and organs. GDP has been linked to the subunit of G - inactive.
The family of human GPCRs consists of the subfamily of the RNA class (ah) in the RNA sequence. About 350 non-olfactory members are considered drug-able from 826 GPCRs and 165 are validated drug targets (Fig.
Mutations within the gene subunit G protein are identified that cause constitutive activation and cause death or demise. This gene mutation is a part of the pathogenic pathogenesis for numerous diseases including McCuneAlbright syndrome, a rare hereditarily diagnosed otodystrophy causing the endocrine fibrosis sporadic.