Explore our custom membrane protein production services
KACTUS offers innovative, custom solutions for studying membrane proteins by displaying multipass transmembrane proteins on Virus-Like Particles (VLPs) or nanodiscs, which provide a stable and physiologically-relevant environment for membrane proteins. The full-length membrane proteins are displayed in natural phospholipid bilayers with proper folding and exposed extracellular domains, enabling effective immunization and functional assays.
Service Highlights
Full-Length Proteins
Virus-like Particle or Nanodisc Display
Biotinylation & Fluorescence
Mammalian Expression
6-8 Week Production
Bioactivity Analysis
Looking for a catalog product?
We also offer transmembrane proteins displays on VLPs and Nanodiscs in our off-the-shelf catalog. These solutions provide membrane proteins with high reproducibility and stability with short lead times.
Request Quote:
Areas of Expertise
We have successfully expressed a variety of full-length transmembrane proteins using our VLP & Nanodisc multi-transmembrane protein expression systems. Our expertise includes:
GPCRs
- GPRC5D
- GLP-1R
- GIPR
- CCR2b
- CCR4
- CCR7
- CCR8
- A2AR
- Cannabinoid Receptor
- SSTR2
- CXCR4
- CXCR5
- CX3CR1
- LGR4
- LPAR1
- EDNRA
- GCGR
- MRGPRX2
4HB/Tetraspanins
- Claudin18.2
- Claudin 9
- Claudin 6
- Claudin 4
- Claudin 3
- TM4SF1
- TM4SF15
- CD9
Solute Carriers
- SLC6A17
- SLC7A1
- SLC7A11
Others
- STEAP1
- CD20/MS4A1
- CD133
Don't see your protein listed? We love a challenge!
Let us know what difficult-to-express protein you're interested in and we'll let you know the feasibility.
Product Formats
Virus-like particle (VLP):
We leveraged our well-established VLP platform to display transmembrane proteins to support your biologics research and discovery against these challenging targets. This robust membrane protein displaying format are highly recommended for animal immunization and is also compatible to bioassays, including ELISA and SPRs. Biotinylation and fluorescent labeling of VLPs are also available upon request.
Nanodisc:
Our SMA-based nanodisc system displays multi-pass transmembrane proteins in a detergent-free process, making it a versatile tool for your membrane protein research. Nanodiscs can be widely applied in antibody panning, antibody screening, PK/PD studies, and analytical assay development/testing such as ELISA, SPR, and BLI. Additionally, we can customize the nanodisc based on your needs, including biotinylation, tag selection, and fluorescent labeling.
Case Studies
STEAP1 VLP
KACTUS has successfully developed full-length STEAP1, a challenging membrane protein target implicated in cancer progression and iron metabolism. STEAP1, a six-transmembrane epithelial antigen of the prostate, is highly expressed in various tumors and is a promising therapeutic target. We have produced functional STEAP1 protein displayed on VLPs.
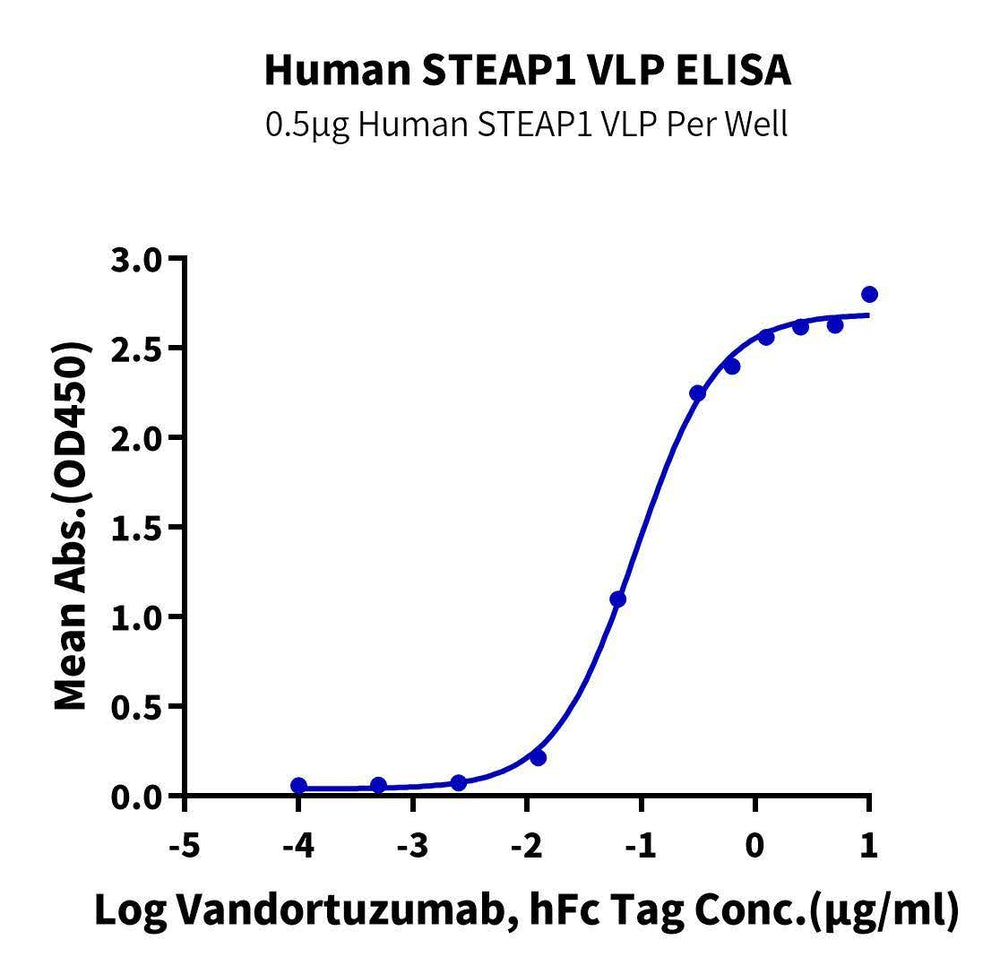
Figure 1. Immobilized Human STEAP1 VLP at 5 ug/ml (100ul/well) on the plate. Dose response curve for Vandortuzumab with the EC50 of 0.21ug/ml determined by ELISA.
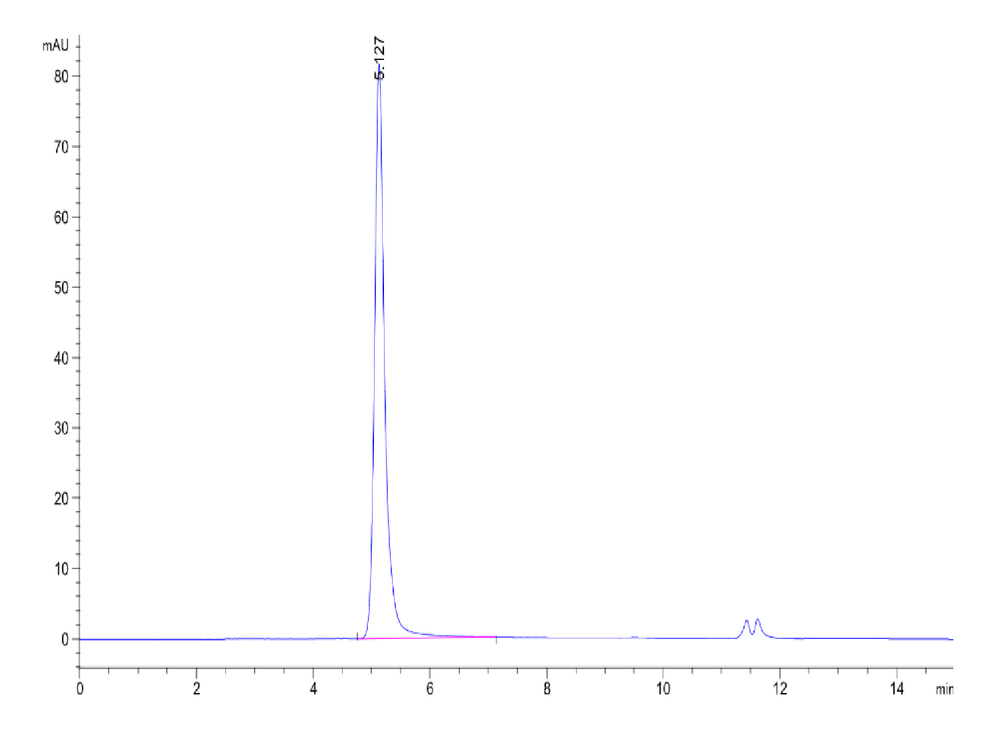
Figure 2. The purity of Human STEAP1 VLP is greater than 95% as determined by SEC-HPLC.
SLC13A5 Nanodisc
KACTUS has successfully developed full-length SLC13A5, a challenging drug discovery target known for its six transmembrane domains. SLC13A5, a sodium-coupled citrate transporter, plays a vital role in cellular metabolism and energy production. We have created an SLC13A5 copolymer nanodisc with detergent-free extraction.
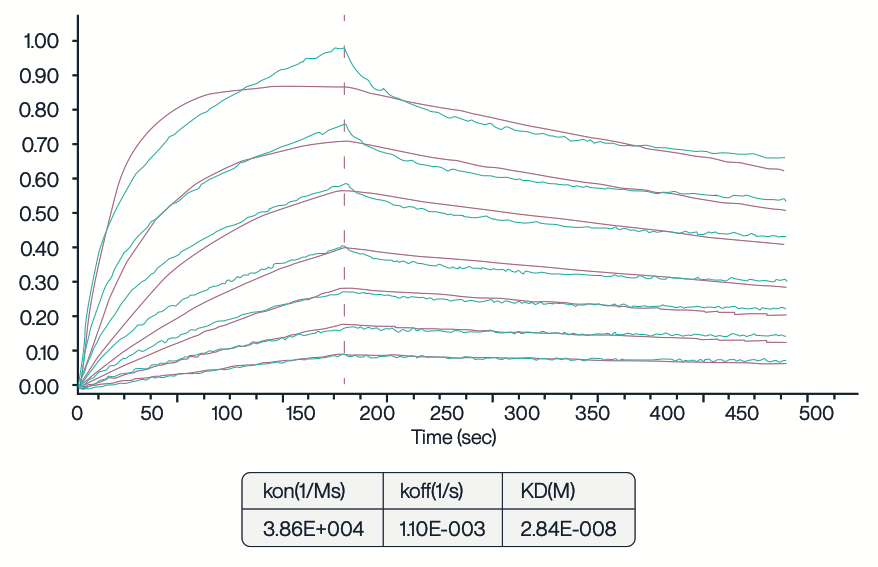
Figure 4. Full-length SLC13A5 nanodisc was tested using BLI. The His-tagged SLC13A5 nanodisc was immobilized on an Anti-His chip, and the binding interaction was analyzed using the 11H7B8 antibody at varying concentrations: 1023.33, 511.67, 255.83, 127.92, 63.96, 31.98, and 15.99 nM. The KD value of 28.4 nM indicates a high affinity of the SLC13A5 nanodisc for the 11H7B8 antibody.
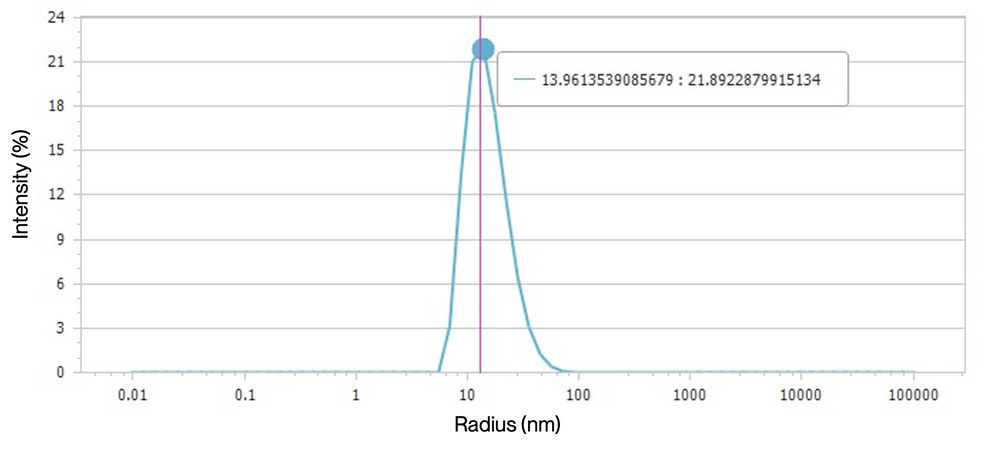
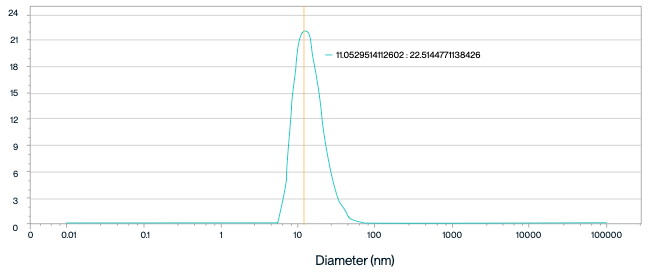
Figure 5. Dynamic Light Scattering (DLS) Analysis of Human SLC13A5 Nanodiscs. The peak is centered around a radius of 13.96 nm, with a corresponding intensity of 21.89%, suggesting that the Human SLC13A5 nanodiscs are predominantly monodisperse, with minimal aggregation.
PC2 Nanodisc
Our team successfully expressed full-length Polycystin-2 (PC2), a calcium channel that can be found in the endoplasmic reticulum, the plasmatic membrane, and the primary cilium. We have developed a custom Human PC2 Nanodisc expressed from HEK293 and validated its activity via ELISA.
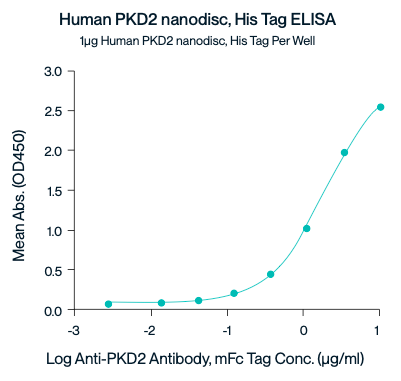
Figure 7. Immobilized Human PC2 nanodisc, His Tag at 10ug/ml (100ul/well) on the plate. Does response curve for Anti-PC2
Antibody, mFc Tag with the EC50 of 3.34 ug/ml determined by ELISA.
Why Choose KACTUS for Membrane Protein Expression?
Expertise in Challenging Membrane Protein Expression
At KACTUS, we have spent years tackling complex recombinant membrane protein expression including difficult targets like Claudin 18.2 and CCR8. With deep experience in optimizing expression conditions, solubilization strategies, and proper folding, our team is knowledgeable in how to optimize expression conditions, solubilization, and bioactivity for your complex target.
Advanced Membrane Protein Display Platforms
Our mature membrane protein display formats maintain membrane protein conformation in a native-like environment. After expressing a variety of membrane proteins including GPCRs, SLC proteins, and ion channels on these platforms, we understand how to utilize them to product accurate target proteins for various applications.
Bioactivity validation and quality control
Each membrane protein we produce undergoes quality control validation to ensure structural integrity and functional activity. Our quality control processes include cell sorting, binding assays, and purity testing to give you full confidence in the product quality.
Transparent, responsive scientific support
With KACTUS, you can work with scientists who understand your challenges and care about your results. We’re committed to transparent communication, timely updates, and collaborative problem-solving—so you can move forward with clarity and confidence.
Explore our most popular custom solutions
Custom Transmembrane Proteins FAQs
Virus-Like Particles (VLPs) are non-infectious, self-assembling membrane spheres that mimic the structure of viruses but lack any genetic material. At KACTUS, we use VLPs to display full-length transmembrane proteins in a native-like lipid bilayer, preserving correct protein folding and extracellular domain orientation. This makes them highly suitable for immunization in antibody discovery, ELISA, and SPR assays.
VLPs are preferred for cell-based assays, antibody screening, and immunization, while nanodiscs are ideal for biophysical characterization, in vitro assays, and situations requiring detergent-free environments. Feel free to reach out, and we’ll help you determine the most appropriate platform based on your application.
We can express a wide range of difficult targets, including GPCRs, solute carrier (SLC) transporters, and tetraspanins. Examples include Claudin 18.2, CXCR4, SLC13A5, and GPRC5D, with validated formats across both display systems. Ion channels are better suited for nanodiscs.
Please submit your target protein name or sequence, intended application, preferred display format (VLP or nanodisc), and any customization requests such as tags, labels, or buffer conditions. Our team will assess feasibility and timeline.
Custom membrane protein expression projects are typically completed within 6–8 weeks including shipping, depending on protein complexity and required modifications.
We use HEK293 cells for their ability to support proper folding and post-translational modifications, ensuring biologically active, full-length membrane proteins.
Each construct undergoes rigorous quality control, including ELISA, SPR, BLI, FACS, DLS, or HPLC, based on your intended use. We ensure structural integrity and functional performance.
Yes. We support biotinylation, fluorescent labeling, and addition of His-tags or other affinity handles to suit your assay requirements.
No problem. We specialize in difficult-to-express targets and welcome custom requests. Share your protein of interest and we’ll evaluate feasibility based on expression history and platform compatibility.
You will receive the purified membrane protein, a Certificate of Analysis, and any requested QC data. Our scientific team will also be available for ongoing support throughout your project.