Ensuring Safe and Effective Gene Therapy with Precise AAV Capsid Quantification
By Mallory Griffin
KACTUS’ AAV Titration ELISA Kits utilize monoclonal antibodies specific to individual serotypes for precise titration of intact AAV capsids.
About AAV Gene Therapy
The delivery systems in gene therapy are primarily classified into two categories: viral vector delivery and non-viral vector delivery. Due to the higher delivery efficiency and stronger tissue specificity, viral vector delivery is, of no doubt, the preferred major approach for gene delivery.
The common viral vectors that have been used for gene delivery include adeno-associated virus (AAV), adenovirus, lentivirus, and retrovirus. Among these viral vectors, AAV stands out as the dominant choice in gene therapy given its excellent transduction efficiency and relatively reliable safety profile. Currently, AAV vectors are widely utilized across investigational drugs in preclinical & clinical stages, as well as gene therapy drugs that have been successfully approved and commercialized. Globally, eight AAV-based gene therapy drugs have been approved for commercialization so far.
|
Drug Name |
Approval Year |
Company |
AAV Serotype |
Indication |
|
Glybera |
2012 |
uniQure |
AAV1 |
Lipoprotein lipase deficiency (LPLD) |
|
Luxturna |
2017 |
Spark/Roche |
AAV2 |
RPE65 mutation-associated inherited retinal dystrophy |
|
Zolgensma |
2019 |
Novartis |
AAV9 |
Spinal muscular atrophy (SMA) |
|
Hemgenix |
2022 |
CSL Behring/uniQure |
AAV5 |
Hemophilia B |
|
Roctavian |
2022 |
BioMarin |
AAV5 |
Hemophilia A |
|
Upstaza |
2022 |
PTC Therapeutics |
AAV2 |
Aromatic L-amino acid decarboxylase deficiency (AADC deficiency) |
|
Elevidys |
2023 |
Sarepta/Roche |
AAVRh74 |
Duchenne muscular dystrophy (DMD) |
|
Beqvez |
2024 |
Pfizer |
AAVRh74var |
Hemophilia B |
Table 1. AAV-based gene therapy drugs in the commercial stage.
ELISA-based Method for AAV Capsid Titer Measurement
With the rapid development of the AAV-based gene therapy market, regulatory agencies have established stricter requirements for quality control (QC) testing methods for AAV drugs. To ensure the safety and efficacy of AAV therapeutics, it is crucial to develop an efficient, reliable, and stable method for quantifying the titer of AAV viral particles.
Various methods are currently available for AAV viral particle titer quantification, including enzyme-linked immunosorbent assay (ELISA), transmission electron microscopy (TEM), high-performance liquid chromatography (HPLC), and analytical ultracentrifugation (AUC), each with their own advantages and limitations. Among these, ELISA is most widely used due to its high reproducibility, strong specificity, and ease of operation, and is also the method recommended by regulatory agencies for AAV capsid titer quantification.
The ELISA-based quantification utilizes the sandwich enzyme-linked immunosorbent assays to measure the AAV capsid titer in samples. This process involves pre-coating a 96-well plate with AAV monoclonal antibodies while still maintaining the antibody activity. When the capsid standard or testing samples are added, the AAV capsid will specifically bind to the coated antibodies on the plate. A detection antibody and an HRP-conjugated marker are then sequentially added, forming an antibody-antigen-[detection antibody]-[HRP marker] complex. Excessive detection antibody and HRP marker will be removed through washing steps before proceeding to color development. In the development step, HRP catalyzes a colorimetric reaction with the added substrates, where the color intensity is proportional to the AAV capsid titer. The reaction is terminated using a stop solution, and the absorbance at 450 nm is measured using a microplate reader. Based on these absorbance values and the 8-point standard curve, the AAV capsid titer in an unknown sample can be accurately calculated.

Figure 1. Sandwich ELISA detection method for AAV capsid titer.
AAV Serotype-Specific Titration ELISA Kits
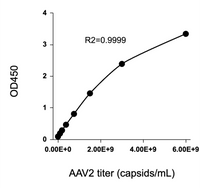
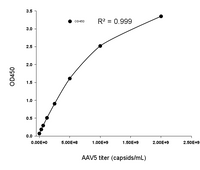
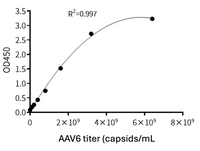
To accommodate the usage of different AAV serotypes in gene therapy research, KACTUS has developed a series of AAV Titration ELISA Kits. These kits enable precise quantification of virus particles for specific serotypes, meeting the diverse needs of research and applications.
Product Advantages
-
Recombinant Antibody Expression Technology. Antibodies are produced using recombinant expression technology, ensuring high reproducibility and consistency with minimal batch-to-batch variation.
-
High Sensitivity & Broad Linear Range. High detection sensitivity and wider linear range, fulfilling the needs of measuring various sample concentrations while ensuring data accuracy.
-
Strong Resistance to Matrix Interference. Excellent resistance to matrix interference, allowing reliable detection even in complex sample environments.
-
High Specificity. Exclusive to the target AAV serotype. Does not interact with denatured AAV or cross-react with other AAV serotypes, ensuring highly specific detection.
-
High Precision & Accuracy. High reproducibility, with intra- and inter-assay CV values below 10%. Spike recovery rates range from 80% to 120%, ensuring accurate measurement and data reliability.
Assay Performance Data
All of our AAV Titration ELISA Kits have been tested and validated for strong assay performance. The following data highlights the AAV9 kit as an example.
Standard Curve with Broad Linear Range
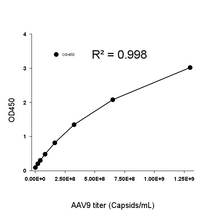
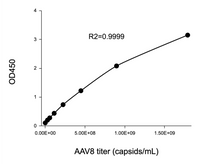
This ELISA kit offers a broad quantification range of 2.03E+07–1.30E+09 capsids/mL (Figure 2).

Figure 2. Example standard curve for AAV9 Titration ELISA kit.
Specificity to Serotypes and Intact Capsids
An indirect ELISA method was used to assess the cross-reactivity between different concentrations of AAV9 detection antibodies and AAV2/5/8/9. (Figure 3). These results demonstrated that the AAV9 detection antibody binds specifically to AAV9 and does not cross-react with AAV2, AAV5, or AAV8, suggesting high specificity of the AAV9 Titration ELISA Kit.

Figure 3. Cross-reactivity of AAV9 detection antibody with AAV2/5/8. The antibody concentration was plotted on the x-axis, and the OD450 value was plotted on the y-axis
The same method was further used to evaluate the binding of the AAV9 detection antibody to intact AAV9 and denatured AAV9. (Figure 4). The AAV9 detection antibody bound specifically to intact AAV9 and did not recognize denatured AAV9 (Figure 4), further confirming the high specificity of the AAV9 Titration ELISA Kit.

Figure 4. Binding specificity of AAV9 detection antibody to intact and denatured AAV9.The antibody concentration was plotted on the x-axis, and the OD450 value was plotted on the y-axis
High Accuracy
The same AAV9 ELISA kit was used to test three different concentrations (high, medium, and low) of AAV9 samples after dilution to evaluate the spike recovery rate. As shown below, the spike recovery rates for AAV9 samples at different titers ranged between 80% and 120% (Figure 5), demonstrating the high accuracy of this ELISA kit for AAV9 titer quantification.

Figure 5. Spike Recovery Rate of Different AAV9 Sample Concentrations in Dilution Buffer.
High Precision
The same batch of ELISA kits was used to test three different concentrations (high, medium, and low) of AAV9 samples. Each sample was tested 10 times, and the mean titer (M), standard deviation (SD), and coefficient of variation (CV%) were calculated.
|
Sample Name |
Repeats (n) |
Mean Titer (M) (capsids/ml) |
Coefficient of Variation (%CV) |
|
AAV9 Sample 1 (6.5E+08 capsids/ml) |
10 |
6.00E+08 |
2.60% |
|
AAV9 Sample 2 (1.63E+08 capsids/ml) |
10 |
1.56E+08 |
1.80% |
|
AAV9 Sample 3 (4.06E+07 capsids/ml) |
10 |
3.65E+07 |
3.60% |
Table 2. Intra-Assay Precision Validation Data.
These results show that the CV values for all three AAV9 sample concentrations were below 10% (Table 2), suggesting that the AAV9 Titration ELISA Kit has excellent intra-assay reproducibility.
Three different batches of the ELISA kit were used to test three different concentrations (high, medium, and low) of AAV9 samples. Each sample was tested 10 times per batch, with a total of 24 tests per sample across the three batches. The mean titer (M), standard deviation (SD), and coefficient of variation (CV%) were calculated.
|
Sample Name |
Repeats (n) |
Mean Titer (M) (capsids/ml) |
Coefficient of Variation (%CV) |
|
AAV9 Sample 1 (6.5E+08 capsids/ml) |
3×10 |
6.14E+08 |
3.40% |
|
AAV9 Sample 2 (1.63E+08 capsids/ml) |
3×10 |
1.54E+08 |
3.10% |
|
AAV9 Sample 3 (4.06E+07 capsids/ml) |
3×10 |
3.66E+07 |
4.90% |
Table 3. Inter-Assay Precision Validation Data.
These results show that the CV values for all three AAV9 sample concentrations across three batches were below 10%, indicating excellent inter-assay reproducibility.
Summary
KACTUS’ AAV Titration ELISA Kits, with their outstanding performance and broad applicability, provide strong support for gene therapy research and development, enabling pharmaceutical companies to advance their AAV gene therapy projects. To learn more or browse our AAV Titration ELISA Kits, please click here or contact support@kactusbio.us.
Product List
|
Catalog No. |
Product Name |
Size |
|
96 Tests |
||
|
96 Tests |
||
|
96 Tests |
||
|
96 Tests |
||
|
96 Tests |