The Landscape of GPCR-targeting Antibody Drug Development
By Mallory Griffin
G protein-coupled receptors (GPCRs) are the largest family of membrane proteins and play essential roles in numerous physiological processes, making them attractive—but historically challenging—targets for drug development. While most approved GPCR-targeting therapies are small molecules, antibody-based approaches are gaining momentum due to their superior specificity and versatility. Here we explore the current research and clinical landscape of GPCR antibody drugs, as well as innovative VLP and Nanodisc GPCR protein display platforms that preserve native structure and bioactivity, accelerating biologics research and development.
Characteristics of GPCRs
The core GPCR structure consists of seven transmembrane α-helices (TM1–TM7), with the N-terminus located extracellularly and the C-terminus intracellularly, connected by extracellular loops (ECLs) and intracellular loops (ICLs (Figure 1). The activation of GPCRs relies on ligand-induced conformational changes, which subsequently activate downstream G proteins (such as Gα and Gβγ) or β-arrestin signaling pathways. This then regulates the production of second messengers (e.g., cAMP, Ca²⁺), ultimately influencing cellular functions [2]. Based on sequence homology and functional differences, GPCRs are mainly classified into four groups:
-
Class A (Rhodopsin-like family): The largest class (~80%), including chemokine receptors, amine receptors, etc. Drugs targeting this class account for 94% of all marketed GPCR-targeting drugs.
-
Class B (Secretin/Adhesion family): Includes receptors such as GLP-1R and GCGR, involved in metabolic regulation and a recent focus for diabetes drug development.
-
Class C (Glutamate receptor family): Includes metabotropic glutamate receptors, associated with neuropsychiatric disorders, and structurally characterized by a Venus flytrap-like ligand-binding domain.
-
Class F (Frizzled/Taste2 family): Encompasses receptors related to the Wnt signaling pathway (such as FZD and SMO), playing critical roles in cancer and development.
![Figure 1. Structure of various GPCR families [2].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GPCR_Structure_Figure1_Blog.png?v=1746814675)
Figure 1. Structure of various GPCR families [2].
Disease Associations and Drug Development Potential of GPCRs
Since 2000, with the advances of high-resolution structure and protein function research, GPCRs have been reported to play significant roles in regulating a variety of major physiological processes. The aberrant GPCR activation or inactivation is tightly associated with diseases including cancers, metabolic disorders, neurodegenerative diseases, tissue inflammation, and cardiovascular diseases [3]. Approximately 40% of currently marketed drugs target GPCRs. However, the drug development potential for these targets remains underestimated, as only about 15% of GPCR family members have been thoroughly studied, and a large number of orphan receptors remain overlooked due to limited understanding of their ligands.
![Figure 2. Representative Categories of Human Diseases Caused by G Protein-Coupled Receptor (GPCR) Dysfunction [3].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GPCR_Classes_blog.png?v=1746814741)
Figure 2. Representative Categories of Human Diseases Caused by G Protein-Coupled Receptor (GPCR) Dysfunction [3].
Market Landscape of Antibody Drugs Targeting GPCRs
As of 2025, the FDA has approved 516 small molecule drugs targeting GPCRs, accounting for 36% of all approved drugs and representing 27% of the global pharmaceutical market's annual sales, indicating a long-lasting dominance of small molecules in GPCR drug development. However, challenges including limited specificity, high toxicity and side effects, drug resistance, and short half-lives are driving the pharmaceutical industry to seek new drug modalities that overcome these limitations. Antibody drugs are undoubtedly the most promising modality to move forward.
Core Advantages of Antibody Drugs over Small Molecules
Compared to small molecules, GPCR-targeting antibody drugs offer several distinct advantages:
-
High specificity and low off-target risk: Antibodies bind to extracellular domains (ECDs) or allosteric sites, avoiding cross-reactivity with conserved orthosteric sites.
-
Long duration of action: Antibodies can have half-lives lasting several weeks (e.g., Erenumab requires only once-monthly subcutaneous injections), improving patient compliance.
-
Peripheral action with limited central exposure: Antibodies typically cannot cross the blood-brain barrier, making them well-suited for diseases requiring peripheral-specific regulation (e.g., migraine, metabolic diseases).
-
Engineering versatility: Bispecific antibodies (e.g., simultaneously targeting a GPCR and an immune checkpoint) and antibody-ligand fusion proteins (e.g., GMA102) can enhance therapeutic efficacy.
Approved GPCR-targeting Antibody Drugs and Ongoing Trial Pipelines
Currently, only three GPCR-targeting antibody drugs have been approved globally, but they have demonstrated strong market performance:
-
Mogamulizumab (targeting CCR4): Approved in Japan in 2012 for T-cell lymphoma; global sales reached $420 million by 2024. It has shown synergistic anti-tumor potential when combined with PD-1 inhibitors.
-
Erenumab (targeting CGRPR): Approved in 2018 for migraine prevention; sales exceeded $2 billion by 2024, validating the role of antibodies in neurovascular diseases.
-
Fremanezumab and Galcanezumab (targeting CGRP): After their 2018 launch, they rapidly captured significant market share. Together with Erenumab, the three achieved a combined annual sales of over $5 billion.
In addition to the approved drugs, there are over 170 GPCR-targeting antibody pipelines globally at stages ranging from preclinical to Phase III trials. These projects span 76 different targets, mainly focused on oncology, metabolic diseases, and immune-inflammatory disorders [3], [4], (Table 1). Notably, GMA102 (Glutazumab), an investigational new drug developed by Gmax Biopharma targeting GLP-1R for type 2 diabetes and obesity indications, is proceeding to Phase III trials with promising efficacy and safety profiles. Leronlimab, a CCR5 antagonist owned by CytoDyn Inc, indicated for HIV infection, triple-negative breast cancer (TNBC), and nonalcoholic steatohepatitis (NASH), is also in Phase II/III stages of multiple clinical trials with potential for future commercialization.
![Table 1. Overview of Biologic Drugs Targeting G Protein-Coupled Receptors [5].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GPCR_Drugs_Blog.png?v=1746814790)
Antibody-Drug Conjugates (ADCs) Targeting GPCRs
In 2024, antibody-drug conjugates (ADCs) emerged as one of the most dynamic and rapidly advancing areas within the biologics field. Within the GPCR family, several members have been identified as tumor-associated antigens (TAAs), including GPRC5D. AZD0305, a therapeutic candidate targeting GPRC5D, is poised to enter Phase II clinical trials for the treatment of multiple myeloma. This agent holds promise for providing new therapeutic options for patients outside of existing modalities such as CAR-T therapies and CD3 bispecific antibodies.
Additionally, several other ADCs targeting GPCRs are currently in clinical development, as detailed in Table 2. With the steady advancement of ADC technology platforms, the combination of ADCs and GPCR targets is expected to meet more unmet clinical needs in the future.
![Table 2. Overview of ADC Drug Development Progress Targeting GPCRs [6].](https://cdn.shopify.com/s/files/1/0627/6025/5710/files/GPCR_ADC_Blog.png?v=1746814847)
Table 2. Overview of ADC Drug Development Progress Targeting GPCRs [6].
Technical Challenges in GPCR Antibody Drug Development
GPCR proteins are indispensable reagents throughout antibody development stages, from early screening to later-stage quality control. The seven-transmembrane structural complexity makes it notoriously difficult to obtain GPCR proteins with intact structural integrity and functional activity, impeding the progression of antibody drug research and development. To overcome the challenge, KACTUS offers a series of GPCR catalog products and custom production services based on virus-like particle (VLP) and Nanodisc platforms to fulfill the needs of pharmaceutical companies for high-quality GPCR antigens for drug research and discovery.
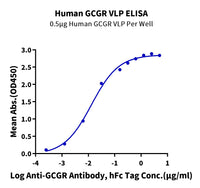
Our VLP protein series utilizes cell membranes to maintain the native conformation of GPCR proteins, preserving activity levels that are theoretically close to those of overexpressed proteins on living cells, including our custom SPR services, supporting reliable affinity characterization in antibody development workflows. These proteins are also suitable for a variety of experimental applications such as SPR, and yeast display (Figure 3, 5). Due to the properties of VLP structures, they can significantly enhance immunogenicity and improve immune responses [7], making them an ideal solution for immunogen development.

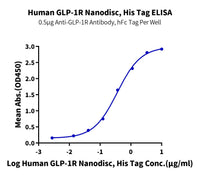
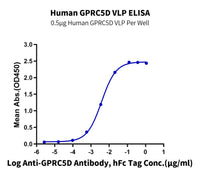
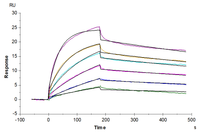
Figure 3. (A) Schematic diagram of GPCR displayed on an enveloped VLP. (B) GPRC5D VLPs are approximately 150nm diameter imaged using transmission electron microscope (TEM). (C) Immobilized Anti-GPRC5D Antibody (2µg/mL with 100µL/well) can bind various dilutions of Biotinylated Human GPRC5D VLP. (D) Biotinylated Human GPRC5D VLP captured on SA Chip can bind Anti-GPRC5D antibody, hFc Tag with an affinity constant of 0.30 nM in SPR assay.
The Nanodisc protein series similarly utilizes native cell membranes to maintain the phospholipid bilayer essential for preserving GPCR conformation. This avoids the risks associated with detergent-based membrane solubilization, which often leads to incorrect refolding during artificial phospholipid bilayer reconstitution. As a result, Nanodiscs also maintain high bioactivity close to the native state and are suitable for experimental applications such as ELISA, SPR, BLI, and yeast display (Figure 4, 5).

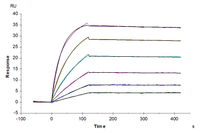
Figure 4. (A) Schematic diagram of GPCR assembled into a copolymer nanodisc. (B) The average size of Nanodiscs measured by DLS. (C) SPR analysis of Human GPRC5D Nanodisc binding against anti-GPRC5D mAb (KD ~ 1.47nM) (D) High binding affinity of Biotinylated Human GPRC5D Nanodisc to anti-GPRC5D mAb (KD = 1.16nM), measured by BLI (Gator).
VLP and Nanodisc GPCR proteins also support advanced antibody development platforms such as phage display panning, yeast display screening (Figures 5A, 5B), and single B cell screening. VLPs additionally support PK (pharmacokinetic) studies and FACS (fluorescence-activated cell sorting) assays (Figure 5D). Moreover, through process optimization and stringent quality control measures, we have achieved effective batch-to-batch consistency (Figure 5C).

Figure 5. FITC-Equivalent GPRC5D VLP (A) and Biotinylated GPRC5D Copolymer Nanodisc (B) were used to screen yeast-displayed rabbit antibodies. (C), GPRC5D VLP activity consistency of three batches was studied with ELISA. (D), FITC-compatible Claudin 18.2 VLP was used to validate the positive rate of HEK293 cell line that overexpresses anti-Cldn18.2 CAR.
GPCR Protein Products & Resources
By harnessing KACTUS’ advanced VLP and Nanodisc membrane protein display platforms, along with deep expertise in structural biology, we continue to drive innovation in protein science to meet the fundamental material needs of biologics research. For more information about GPCRs or other multi-transmembrane targets, please contact us, or browse our GPCR catalog products and resources below.
Learn more about our VLP & Nanodisc platforms
Request custom VLP or Nanodisc protein
Case Study - Application of VLP & Nanodisc GPCR in Yeast Display
Case Study - Application of CXCR4 VLP in Immunization
|
Catalog No. |
Protein |
Species |
Display Platform |
Label |
Tag |
Amino Acid Range |
Expression System |
|
Human |
Nanodisc |
C-His |
Met1-Ser412 |
HEK293 |
|||
|
Human |
VLP |
Met1-Ser412 |
HEK293 |
||||
|
Human |
VLP |
Met1-Leu472 |
HEK293 |
||||
|
Human |
VLP |
Biotinylated |
Met1-Leu360 |
HEK293 |
|||
|
Human |
VLP |
Met1-Leu360 |
HEK293 |
||||
|
Cynomolgus |
- |
C-mFc |
Met1-Lys35 |
HEK293 |
|||
|
Human |
- |
C-mFc |
Met1-Lys35 |
HEK293 |
|||
|
Human |
- |
C-hFc |
Met1-Lys35 |
HEK293 |
|||
|
Human |
Nanodisc |
C-His |
Met1-Leu355 |
HEK293 |
|||
|
Human |
VLP |
Met1-Asn427 |
HEK293 |
||||
|
Cynomolgus |
- |
C-His-Avi |
Gly26-Gln138 |
HEK293 |
|||
|
Cynomolgus |
- |
Biotinylated |
C-His-Avi |
Gly26-Gln138 |
HEK293 |
||
|
Human |
- |
C-hFc |
Gly26-Gln138 |
HEK293 |
|||
|
GIP-HM40R |
GIPR |
Human |
- |
C-His-Avi |
Gly26-Gln138 |
HEK293 |
|
|
Human |
- |
Biotinylated |
C-His-Avi |
Gly26-Gln138 |
HEK293 |
||
|
Human |
Nanodisc |
Biotinylated |
C-His |
Met1-Cys466 |
HEK293 |
||
|
Human |
- |
C-mFc |
Arg24-Glu139 |
HEK293 |
|||
|
Human |
VLP |
Met1-Val345 |
HEK293 |
||||
|
Human |
VLP |
Biotinylated |
Met1-Val345 |
HEK293 |
|||
|
Cynomolgus |
VLP |
Met1-Cys300 |
HEK293 |
||||
|
Mouse |
VLP |
Met1-Leu344 |
HEK293 |
||||
|
Human |
- |
C-His |
Leu28–Leu396 |
HEK293 |
|||
|
Human |
Nanodisc |
C-His |
Ala25-Asp951 |
HEK293 |
|||
|
Human |
- |
C-hFc |
Gly22-Pro543 |
HEK293 |
|||
|
Human |
Nanodisc |
C-His |
Gly22-Pro543 |
HEK293 |
|||
|
Human |
VLP |
Met1-Val364 |
HEK293 |
||||
|
Human |
Nanodisc |
C-His |
Met1-Val330 |
HEK293 |
|||
|
Human |
VLP |
Met1-Ile369 |
HEK293 |
References
-
Yang D, Zhou Q, Labroska V, et al. G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduct Target Ther. 2021;6(1):7. Published 2021 Jan 8. doi:10.1038/s41392-020-00435-w
-
Hutchings CJ. A review of antibody-based therapeutics targeting G protein-coupled receptors: an update. Expert Opin Biol Ther. 2020;20(8):925-935. doi:10.1080/14712598.2020.1745770
-
Zhang M, Chen T, Lu X, Lan X, Chen Z, Lu S. G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery. Signal Transduct Target Ther. 2024;9(1):88. Published 2024 Apr 10. doi:10.1038/s41392-024-01803-6
-
Lorente, J.S., Sokolov, A.V., Ferguson, G. et al. GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov (2025). doi.org/10.1038/s41573-025-01139-y
-
Peterson SM, Hutchings CJ, Hu CF, Mathur M, Salameh JW, Axelrod F, Sato AK. Discovery and design of G protein-coupled receptor targeting antibodies. Expert Opin Drug Discov. 2023 Apr;18(4):417-428. doi: 10.1080/17460441.2023.2193389
-
High P, Carmon KS. G protein-coupled receptor-targeting antibody-drug conjugates: Current status and future directions. Cancer Lett. 2023 Jun 28;564:216191. doi: 10.1016/j.canlet.2023.216191
-
Nguyen B, Tolia NH. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines. 2021 May 13;6(1):70. doi: 10.1038/s41541-021-00330-7