Harnessing the Power of Wnt3a: Promoting Growth and Development of Organoids
Organoids are a three-dimensional model established in vitro that is highly similar to tissues or organs in vivo, and can reproduce the complex spatial morphology of differentiated tissues and the interactions between various cells. They play an important role in the fields of disease modeling, organ regeneration and personalized therapy. The culture of organoids requires the maintenance of various cytokines, among which Wnt3a is one of the most commonly used factors, occupying a key position in the culture of most tissue-derived organoids.
Wnt3a is a member of the Wnt protein family, which can mediate the canonical Wnt-β-catenin signaling pathway by binding to Frizzled protein on the cell membrane and low-density lipoprotein receptor LRP5/6, so that β-catenin can accumulate in the cell and bind to the nucleus The combination of transcription factors LEF/TCF initiates the expression of downstream target genes such as c-Myc and Cyclin D1, and then participates in the regulation of cell development, proliferation, differentiation, adhesion, polarity, intercellular communication, survival and self-renewal, etc. In addition, Wnt3a can also only bind Frizzled to activate the non-canonical Wnt pathway.

Figure 1. Wnt3a signaling pathway [1].
Wnt3a can not only promote the growth, proliferation and differentiation of normal cells, but also participate in the regulation of cell fate and pattern during tumorigenesis and embryonic development. Based on these important functions, Wnt3a as a cytokine has been widely used in primary cell culture research.
Wnt3a Promotes Organoid Growth
Wnt3a has been shown to be an important component for maintaining the proliferation of Lgr5+ stem cells in intestinal epithelial cells. Sato T et al. found that in the presence of substances such as Wnt3a, a single Lgr5+ cell derived from intestinal crypts could be cultured into intestinal organoids [2]. In the high-content Wnt3a medium, most of the cells in the intestinal crypt area were EdU-positive, showing obvious proliferation characteristics, and no differentiation was seen. When Wnt3a was withdrawn, the cells stopped proliferating and differentiated [3].

Figure 2. Wnt3a promotes crypt-villous unit generation in the small intestine. (Top: Without Wnt3a; Bottom: With Wnt3a) [3].
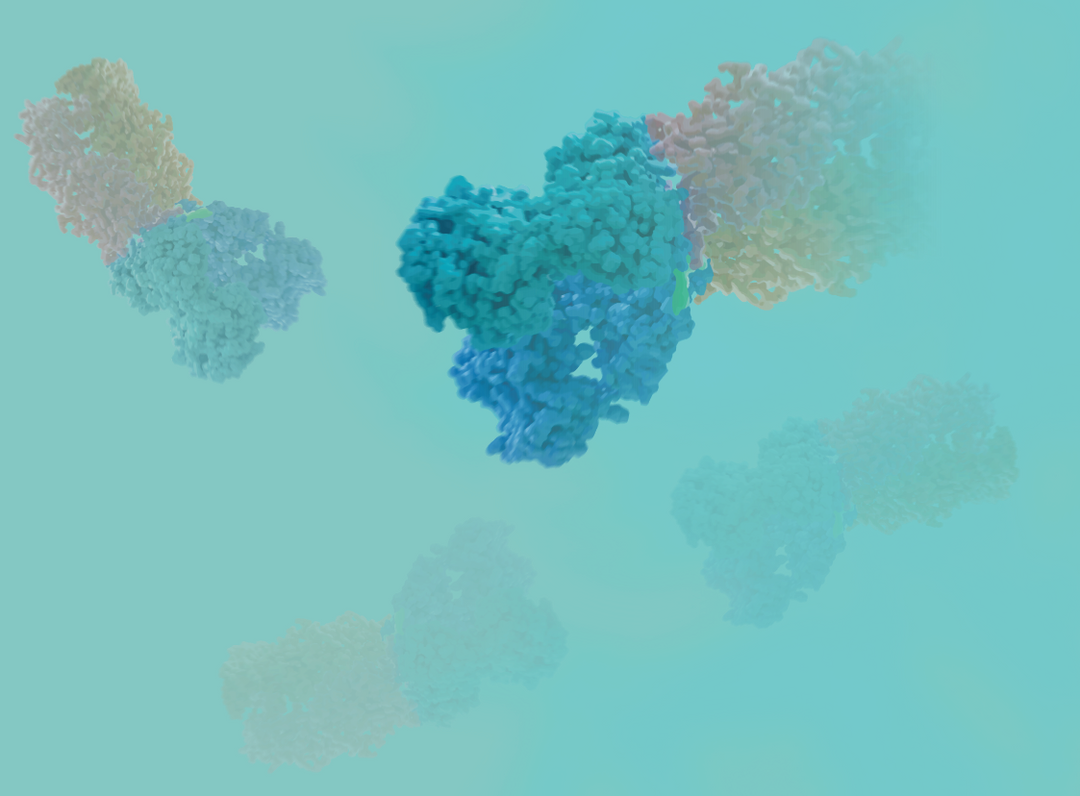
In addition, studies by Bartfeld S. et al. have shown that adding Wnt3a can significantly promote the formation of gastric organoids without karyotype changes [4]. In the establishment of organoid models derived from gastric cancer tissue, higher concentrations of Wnt3a are more effective than lower concentrations [5].

Figure 3. Wnt3a promotes proliferation of gastric organoids [4].
Wnt3a has been used in the culture of various organoids. In addition to the intestinal and gastric organoids described above, it can also be used in the culture of organoids such as pancreas, liver, thyroid, esophagus and nervous system.
In recent years, the field of organoids has continued to heat up. In order to support the development of organoid culture technology, KACTUS has successfully developed a series of organoid cytokine products such as recombinant Wnt3a with high activity and low endotoxicity. The performance is stable and reliable, and can be applied to the cultivation of various organoids and applied research, and at the same time provide stem cells and immune cells and other primary cell culture-related factor products to meet customer needs.
KACTUS Wnt3a can successfully induce the activity of the Topflash reporter gene in HEK293T cells.
KACTUS recombinant Wnt3a protein can successfully induce the activity of the Topflash reporter gene in HEK293T cells, with an ED50 value of 21.02 ng/mL, which is significantly better than competing products.

Order Recombinant Wnt3a now.
References
[1] Volleman TNE, Schol J, Morita K, Sakai D, Watanabe M. Wnt3a and wnt5a as Potential Chondrogenic Stimulators for Nucleus Pulposus Cell Induction: A Comprehensive Review. Neurospine. 2020 Mar;17(1):19-35.
[2] Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011 Nov;141(5):1762-72.
[3] In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):633-642.
[4] Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015 Jan;148(1):126-136.e6.
[5] Wuputra K, Ku CC, Kato K, Wu DC, Saito S, Yokoyama KK. Translational models of 3-D organoids and cancer stem cells in gastric cancer research. Stem Cell Res Ther. 2021 Sep 6;12(1):492.