Overview
Cell therapy includes immunocellular therapy and stem cell therapy. It utilizes cells from the patient or a donor, which, after being cultured, expanded, activated, or genetically edited ex vivo, are reinfused into the patient. This stimulates or enhances the body's immune function, thereby achieving a method of controlling diseases. Based on different mechanisms of action, typical types of cell therapy include CAR-T, TILs, TCR-T, CAR-NK, etc.
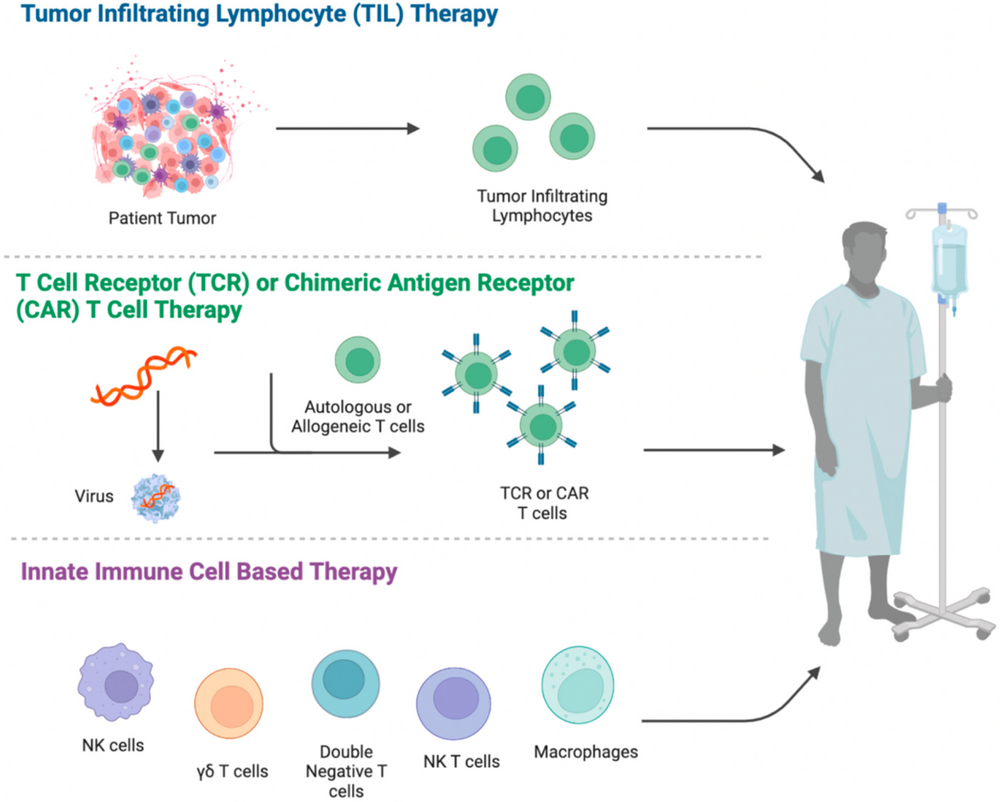
Figure 1. General Process of Immunocellular Therapy [1][2].
Immunization Proteins for Drug Discovery
CAR-T Target Proteins
During the drug discovery phase of cell therapy, especially for reprogrammed cell therapeutic drugs, such as immune cells loaded with CAR (Chimeric Antigen Receptor), it is often necessary to obtain the correct scFv (Single-chain Variable Fragment) sequence through immunization with relevant target antigen proteins. This enables the drug product cells to accurately identify tumor cells expressing antigens. KACTUS offers a series of high-quality target antigen protein options for customers to choose from. KACTUS' high-activity CAR-T target proteins can be applied during the early animal immunization stage for antibody discovery.
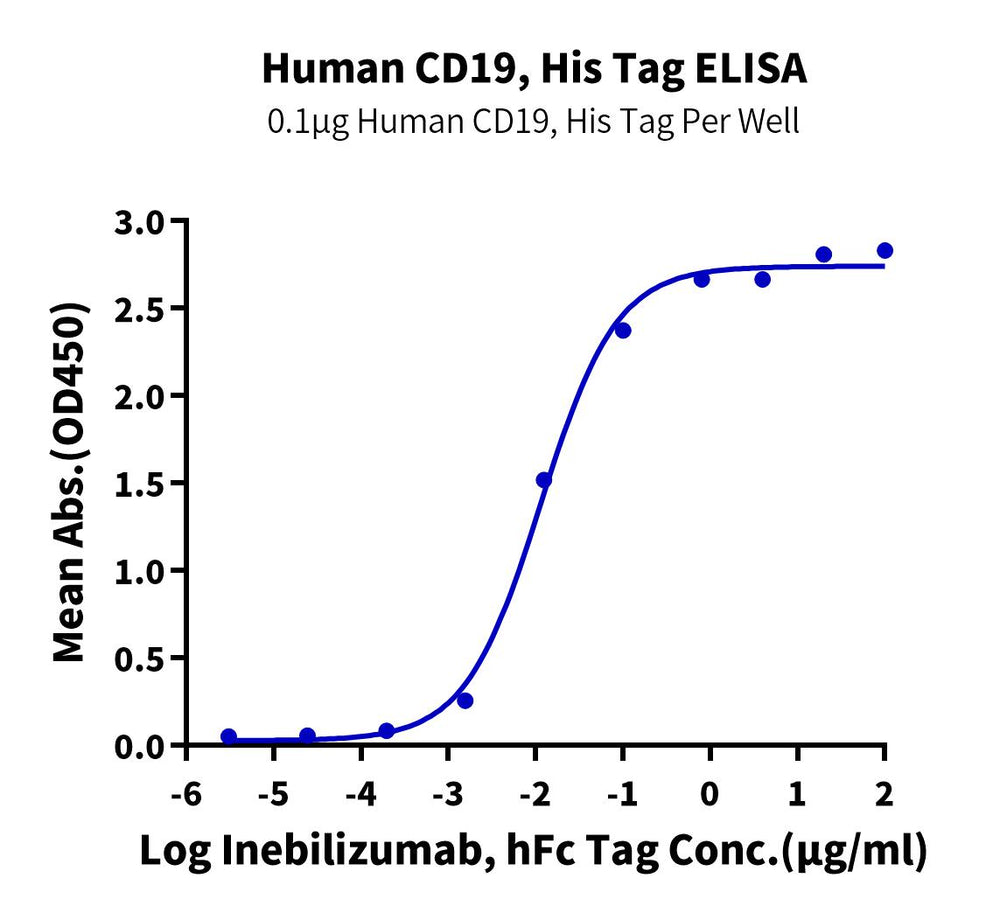

CAR-T target proteins, Human CD19 and Human CD7 proteins, can both bind well with the corresponding antibodies.
MHC Monomers & Tetramers
Intracellular antigen targets are a major direction in cell therapy. Peptide segments, resulted from the degradation of intracellular antigens, form complexes with MHC and are presented on the cell surface for TCR recognition. Targeting MHC peptide complexes can achieve the killing of tumor cells.
KACTUS peptide-MHC complexes cover a variety of popular targets, and include monomers, tetramers, fluorescently-labeled tetramers, and other forms, which can be used for immunization or screening research in the development of T-cell therapeutic drugs. KACTUS also offers custom MHC expression, custom TCR expression, and peptide-ready MHCs to build your own peptide-MHC in house.
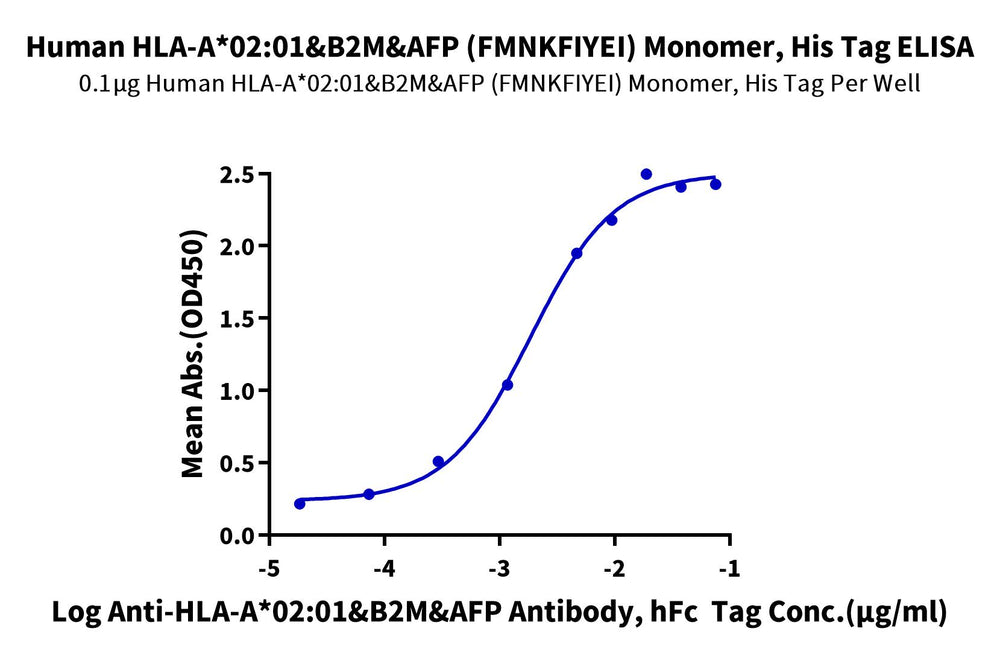
Through ELISA and SPR experiments, we have verified that the monomer of HLA-A*02:01&B2M&AFP (FMNKFIYEI) complex can bind separately with Anti-AFP (HLA-A*02:01) Antibody and HLA-A*02:01&B2M&AFP TCR. The EC50 and affinity constant are 7.6 ng/mL and 0.923 μM, respectively.
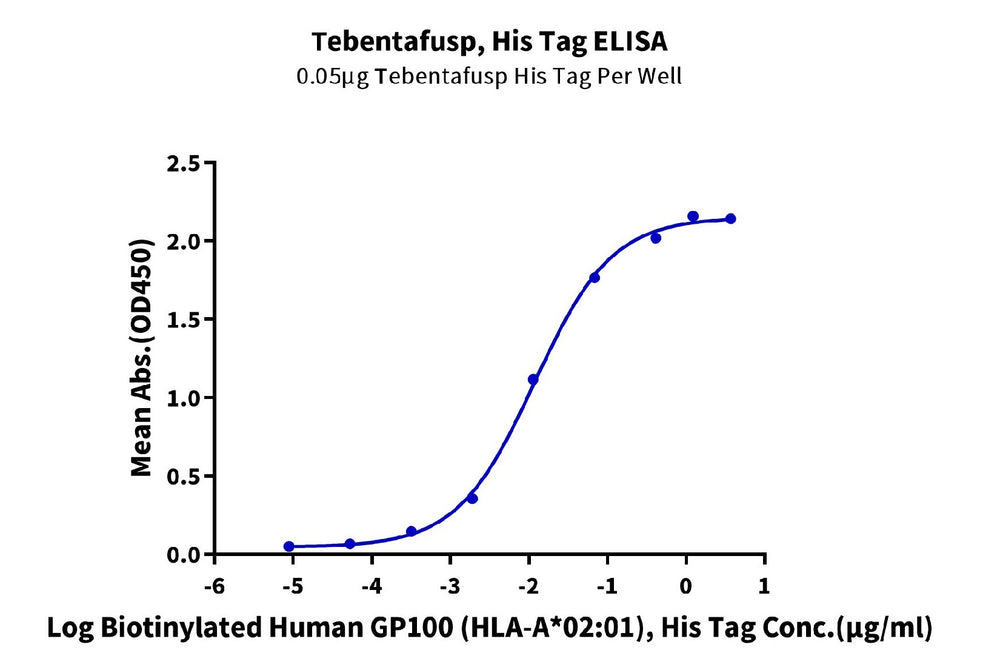
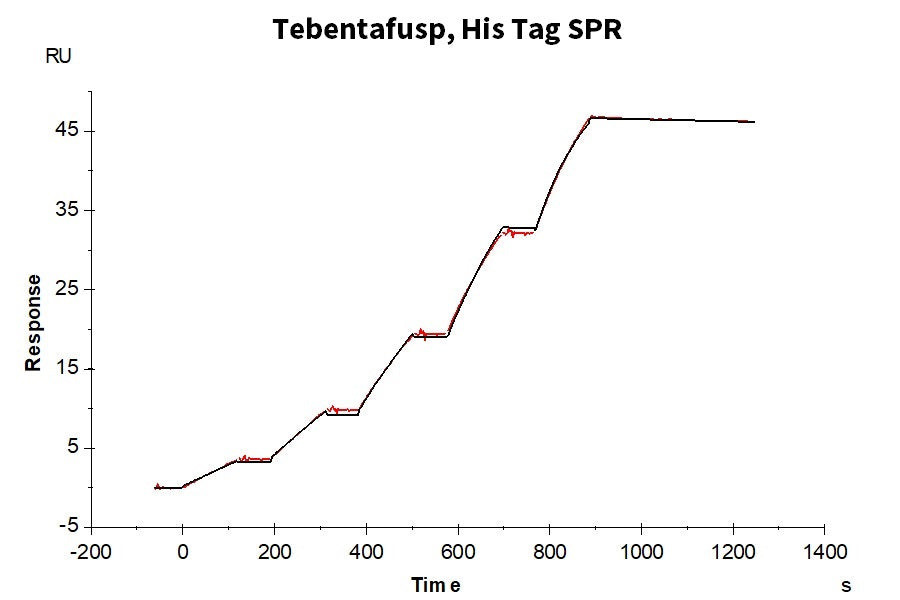
(Left) Through ELISA validation, it has been confirmed that gp100 TCR & CD3 scfv fusion protein can bind to the monomer of HLA-A*02:01&B2M&GP100 (YLEPGPVTA) complex, with an EC50 of 11.8 ng/mL. (Right) In the SPR analysis, gp100 TCR & CD3 scfv fusion protein can bind to the tetramer of the HLA-A*02:01&B2M&GP100 (YLEPGPVTA) complex, with an affinity constant of 0.196 nM.
VLP-Displayed Antigens
Cell therapy targets include multiple transmembrane proteins (such as the GPCR family) and some targets for which it is difficult to obtain ideal antibody sequences through direct immunization. Our virus-like particle (VLP) display technology can maintain the correct conformation of multiple transmembrane proteins while enhancing the immunogenicity of the protein. In addition, the VLP framework can be designed specifically for your antigen with our custom VLP services.
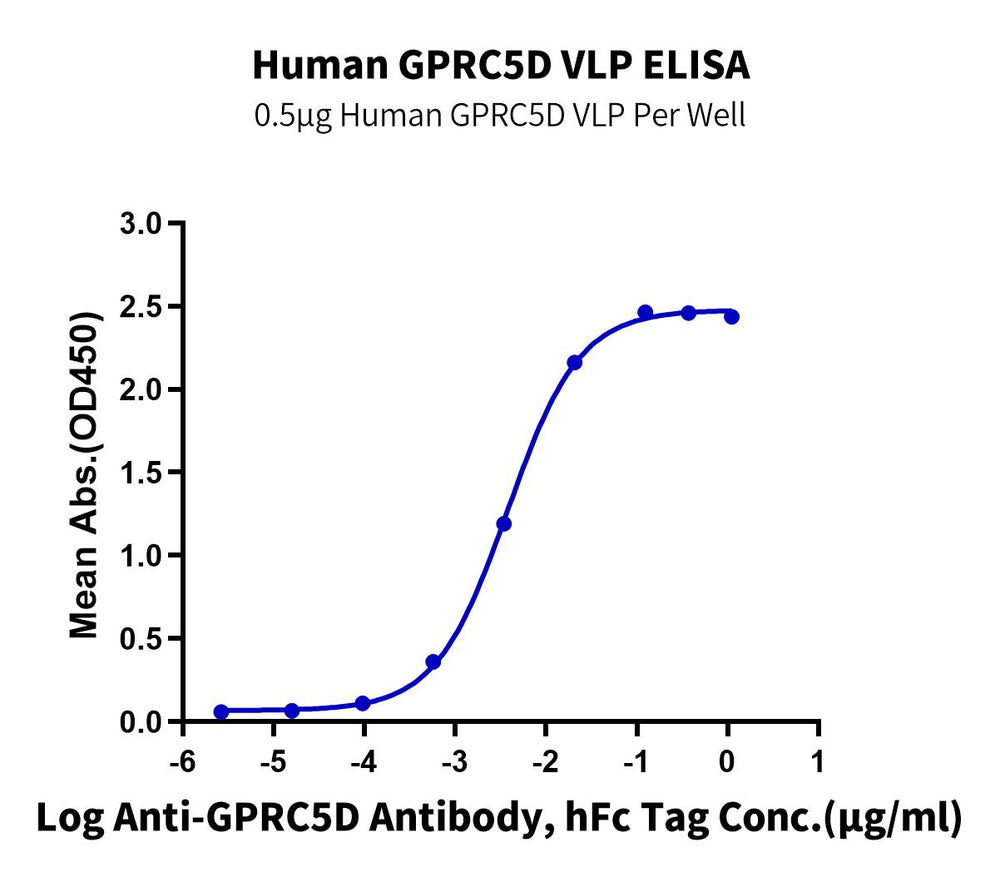
Immobilized Human GPRC5D VLP at 5ug/ml (100ul/Well) on the plate. Dose response curve for Anti-GPRC5D Antibody, hFc Tag with the EC50 of 3.8ng/ml determined by ELISA.
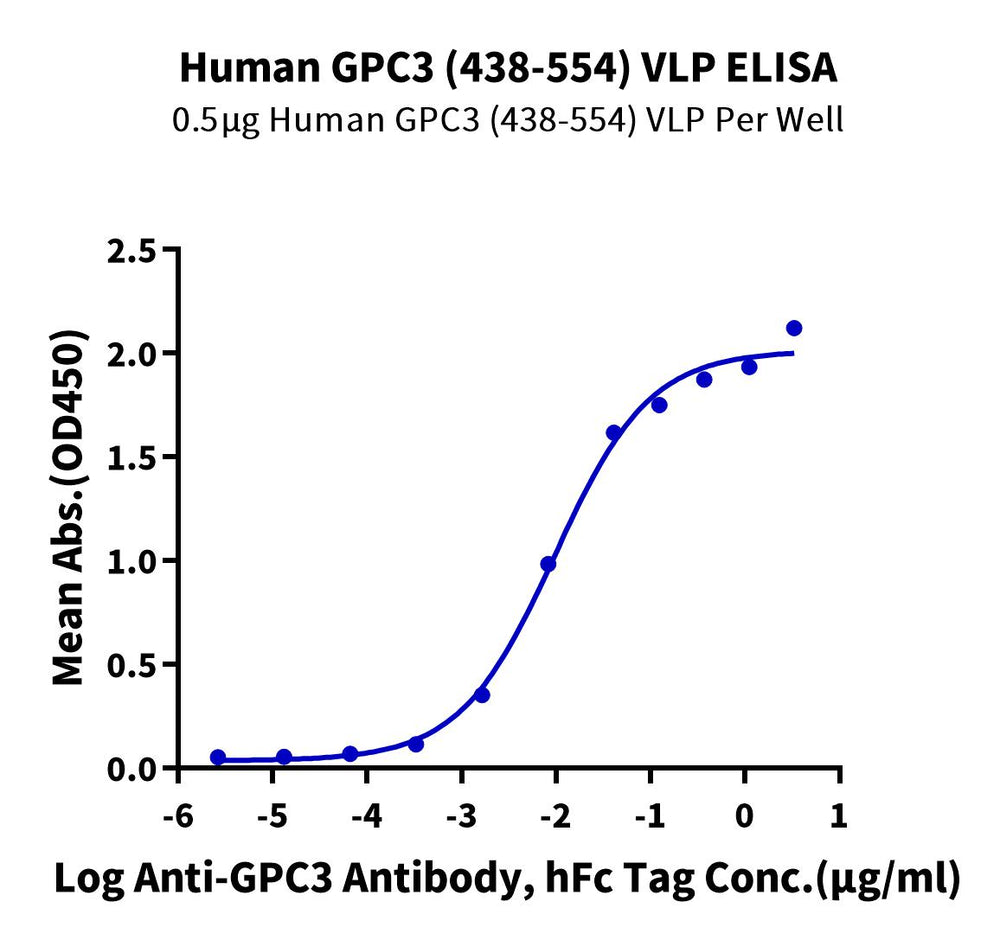
Immobilized Human GPC3 (438-554) VLP at 5ug/ml (100ul/Well) on the plate. Dose response curve for Anti-GPC3 Antibody, hFc Tag with the EC50 of 9.7ng/ml determined by ELISA.
Proteins for Cell Therapy Production & Analysis
Production preparation is a crucial step to obtain immunocellular drugs and is also key to the successful registration of cell drugs. KACTUS provides high-activity cytokines for cell culture and cell matrix proteins required for stem cell differentiation.
Laminin 521
KACTUS offers recombinant Laminin 521 at research-grade and preclinical-grade. Laminin 521 is an important matrix adhesion protein for maintaining stem cells in culture.
The cell culture matrix protein Laminin 521 can maintain the differentiation potential of stem cells.
Cytokines
Cell culture is a necessary step for the preparation of cell-based therapies. Immunotherapy involving T cells, NK cells, and stem-cell derived immunocytes requires the preparation of stem cells such as ESCs, MSCs, and iPSCs. KACTUS offers a variety of cytokine products for cell culture.
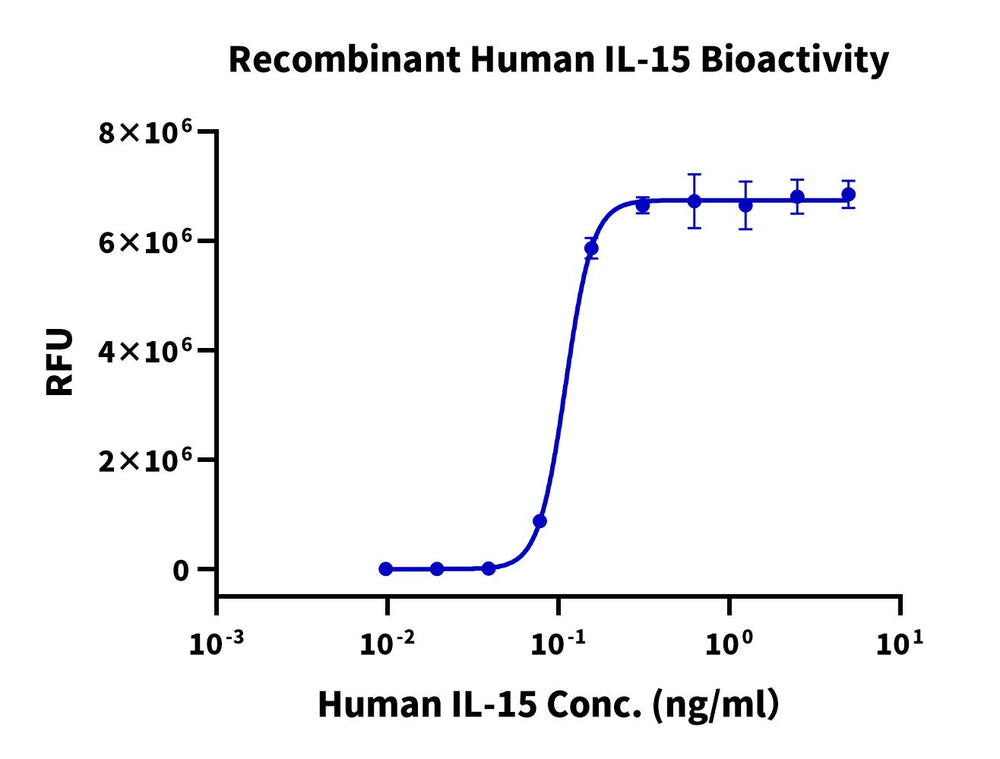
Measured in a cell proliferation assay using CTLL-2 cells. The ED50-for this effect is 0.1 - 0.3 ng/mL. The specific activity of recombinant human IL-15 is > 1 x 10^7-units/mg, which is calibrated against the human IL-15 reference standard (NIBSC code: 95/554) (QC Test).

Measured by its ability to induce Topflash reporter activity in HEK293T human embryonic kidney cells. The ED50-for this effect is 5.2 ng/mL.
Fluorescent or Biotinylated Proteins For Antibody Screening & Cell Therapy Analysis
Proteins labeled with fluorescence or biotin can be used for various detection processes in the production and quality inspection of therapeutic cells, such as antibody screening (such as scFv, etc.), CAR affinity research, CAR-T positivity rate detection, TCR screening or detection, and Blocking Assay, etc. KACTUS provides high-quality proteins labeled with different fluorescent markers like FITC, PE, as well as biotin-labeled proteins, to meet the needs of various analytical scenarios including cellular level analyses.
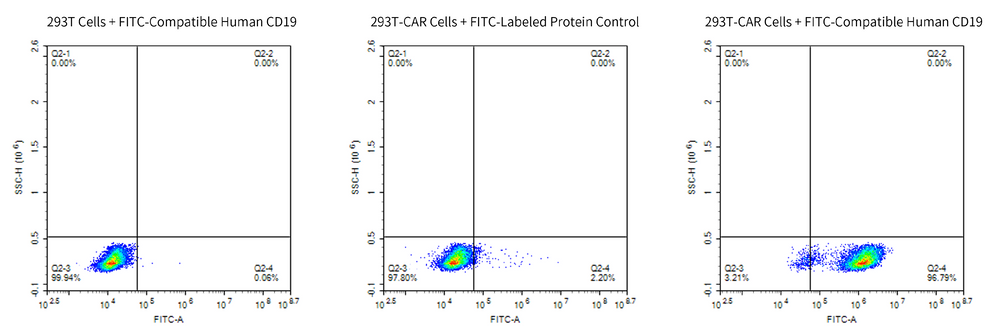
Through cellular-level flow cytometry detection, we demonstrated that FITC-Compatible Human CD19 protein can specifically bind with Anti-CD19-CAR T cells.
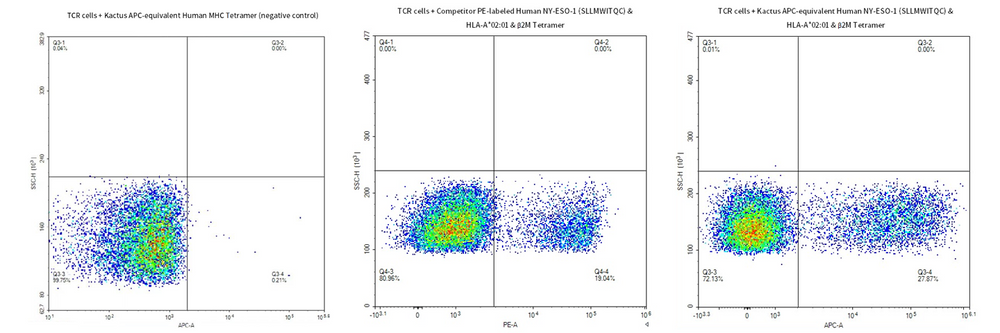
Through cellular-level flow cytometry detection, we demonstrated the fluorescently labeled HLA-A*02:01&B2M&NY-ESO-1 (SLLMWITQC) tetramer complex can bind well with HLA-A*02:01&B2M&NY-ESO-1 TCR cells.
GMP-Grade Gene Editing Enzymes for Cellular Editing
Allogeneic off-the-shelf cellular therapy products, such as UCAR-T, require the use of gene-editing technologies to knock out genes related to immune rejection reactions on donor T cells, to avoid inducing immune rejection reactions like GvHD and HvGR.
KACTUS offers GMP-Grade AccuBase™ Base Editor, GMP-Grade CRISPR Cas9, GMP-Ready Cas12a for genetic modification of cells, including UCAR-T cell therapy. Regulatory support documentation is available. Moreover, our GMP-Grade Cas9 has been registered with the FDA Drug Master files (DMF #036578). Additionally, our CRISPR Cas9 ELISA kit can detect residual Cas9 nuclease in your cell product for various industry-standard Cas9 proteins.
AccuBase™ Base Editor, GMP
Compared to gene editing enzymes, base editing proteins do not introduce double-strand breaks (DSBs) during the editing process, ensuring the safety of the editing. AccuBase™ is a CBE (cytosine base editor) designed through genetic engineering that deaminates C to U within a specific window range. U is then converted to T using the cell's repair mechanisms, thus achieving a C→T conversion. Additionally, the deaminase is innovatively encapsulated in a reversible manner, only activating its deaminating function when the sgRNA binds to the target site. This significantly reduces random binding to non-target DNA, achieving near-zero off-target effects while maintaining high editing efficiency.
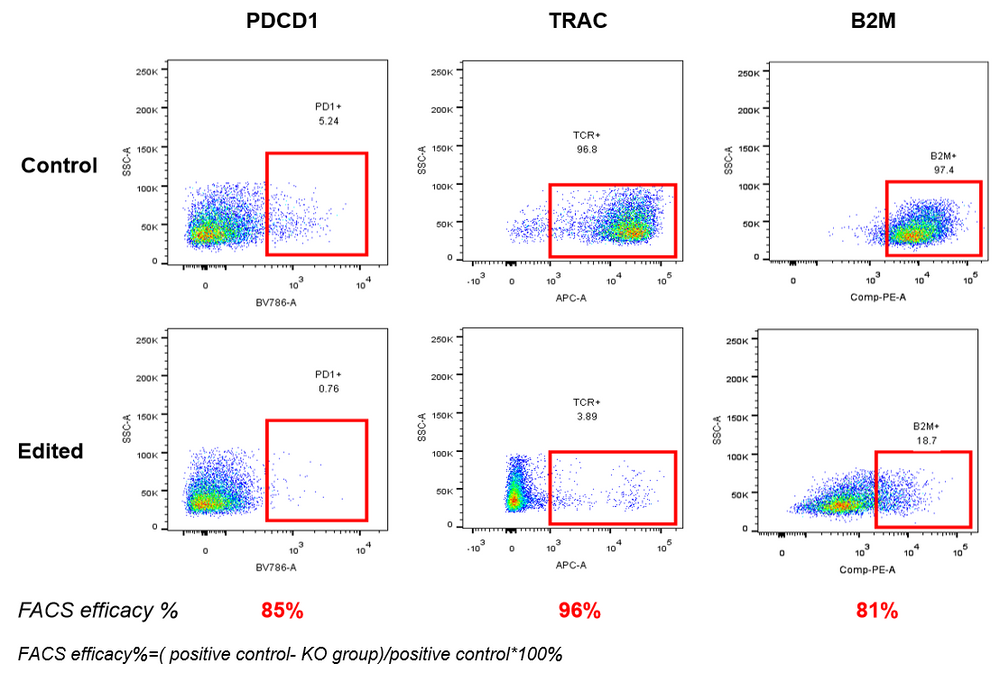
AccuBase™ RNP electroporated primary T cells, detected by flow cytometry, show that AccuBase™ can efficiently knock out PD1, B2M, and TRAC proteins on the membrane of activated primary T cells at the protein level. The knockout efficiency for PD1 and B2M exceeds 80%, and the knockout efficiency for TRAC reaches 96%.
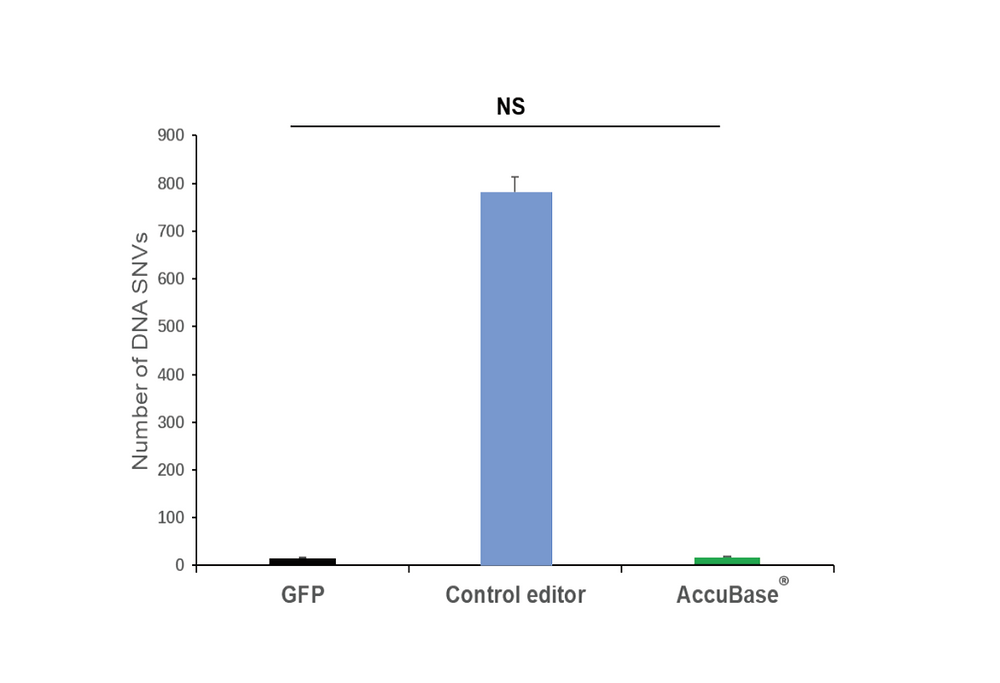
Using GOTI (genome-wide off-target analysis by two-cell embryo injection) to analyze the genome-wide off-target levels of AccuBase®, it was found that compared to the control editor group (which detected over 700 SNVs), the SNVs obtained with AccuBase® were close to the levels of the GFP control group.
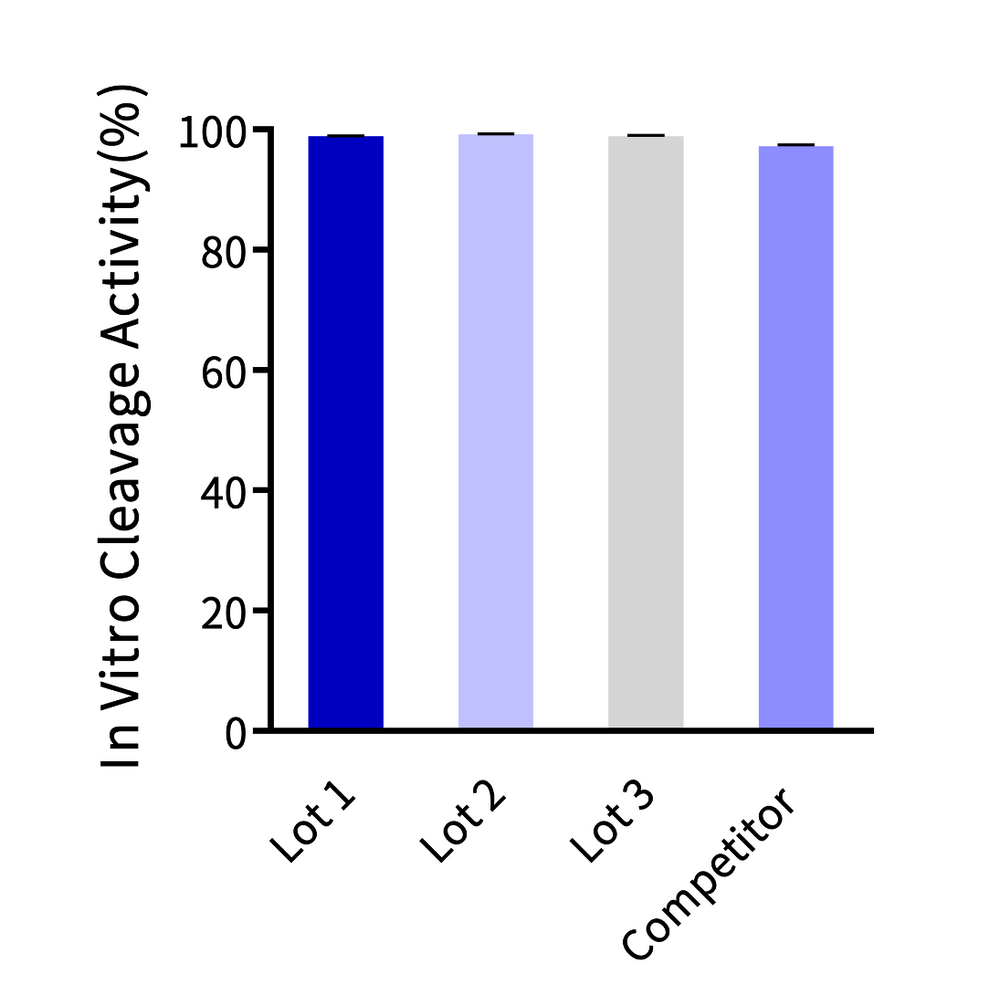
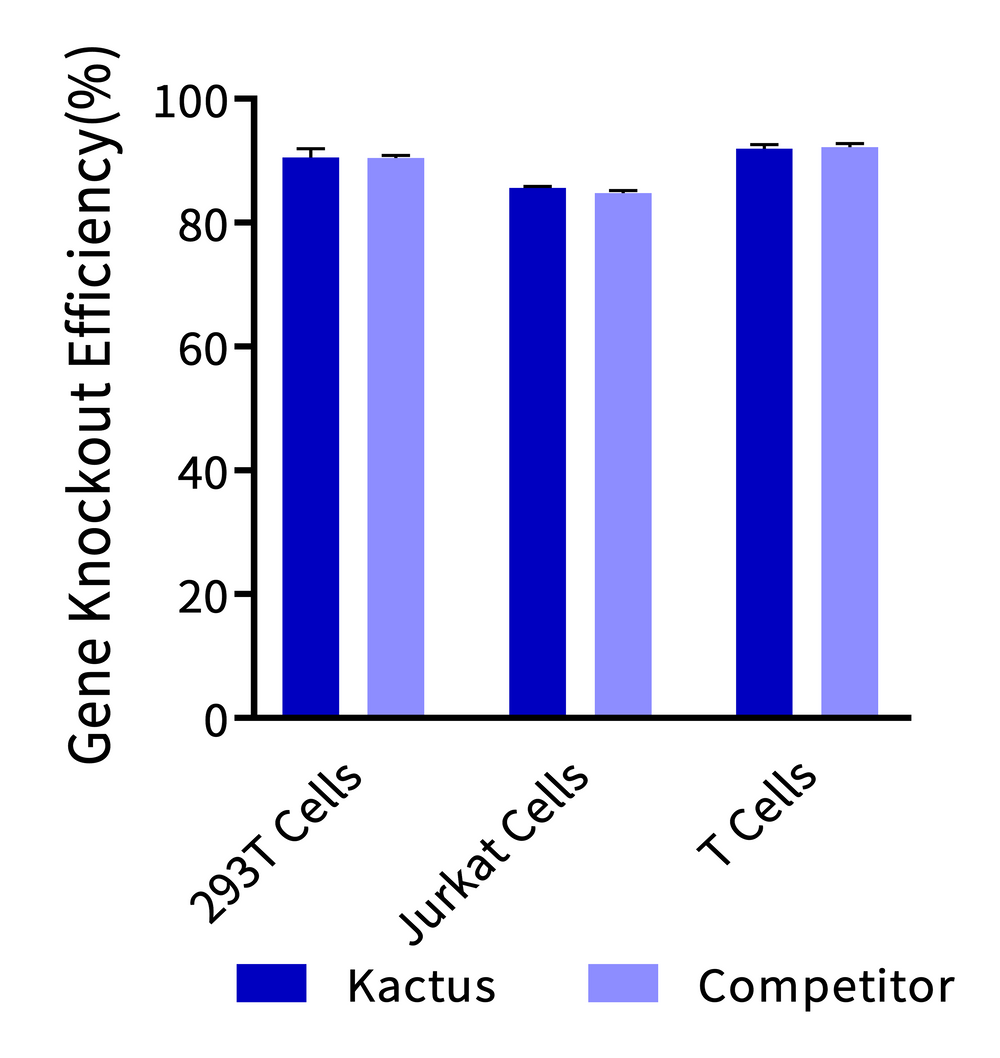
(Left) Cas9 Nuclease is used for gene knockout in 293T cells, Jurkat cells, and T cells, and its gene knockout efficiency in various cell types is comparable to that of leading suppliers. (Right) Cas9 Nuclease In Vitro DNA Cleavage Experiment. The in vitro cleavage efficiency of Cas9 nuclease is equivalent across all three batches.
Universal Cas9 ELISA Kit
Quantitatively detect the residual Cas9 nuclease, either extracellular or intracellular, in gene-edited cell drugs before they are reinfused into the human body. The detection range of the kit is 0.25 ng/ml to 16 ng/ml, with a sensitivity of up to 0.125 ng/ml.
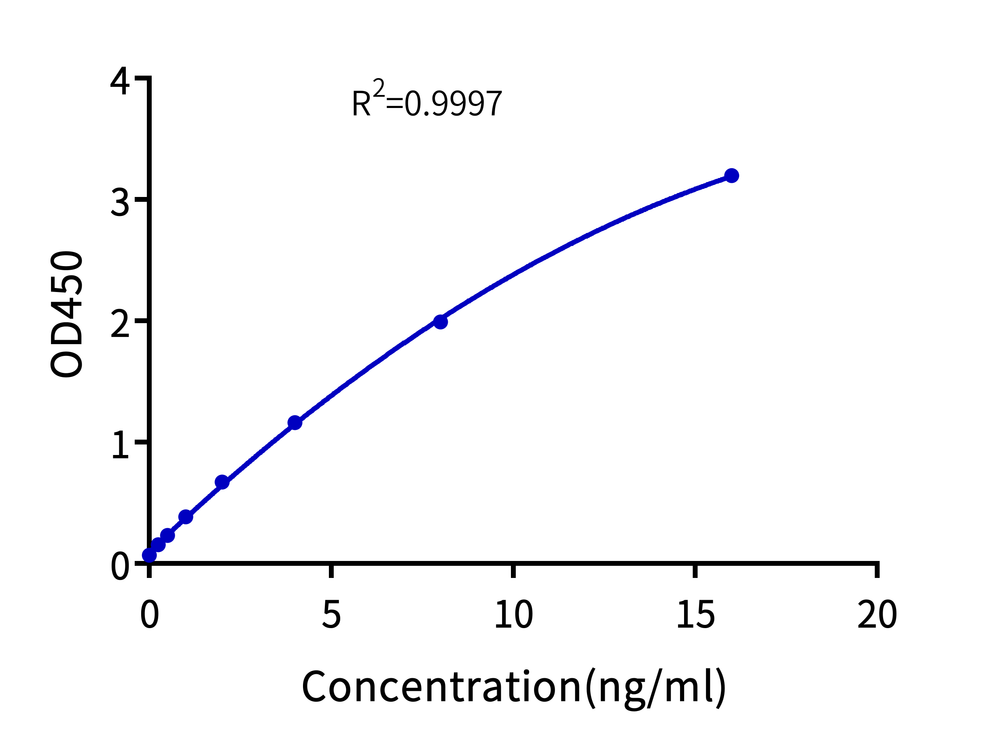
Polynomial fitting of the Cas9 ELISA standard curve. The R² is 0.9997.
MaxNuclease™ for DNA/RNA Degradation, GMP
In gene therapy, MaxNuclease principally serves to eliminate contaminants like host DNA and plasmid DNA present during the pharmaceutical production process. This GMP-grade nuclease is produced within a facility maintaining stringent GMP-Grade production standards, avoiding the use of materials derived from animals. The analytical methods applied have undergone systematic validation, and the final enzyme is subject to extensive quality control release testing to ensure it meets the requirements for use from research and development through to large-scale commercial production. Additionally, KACTUS has submitted MaxNuclease to the FDA Drug Master Files (DMF #036799).
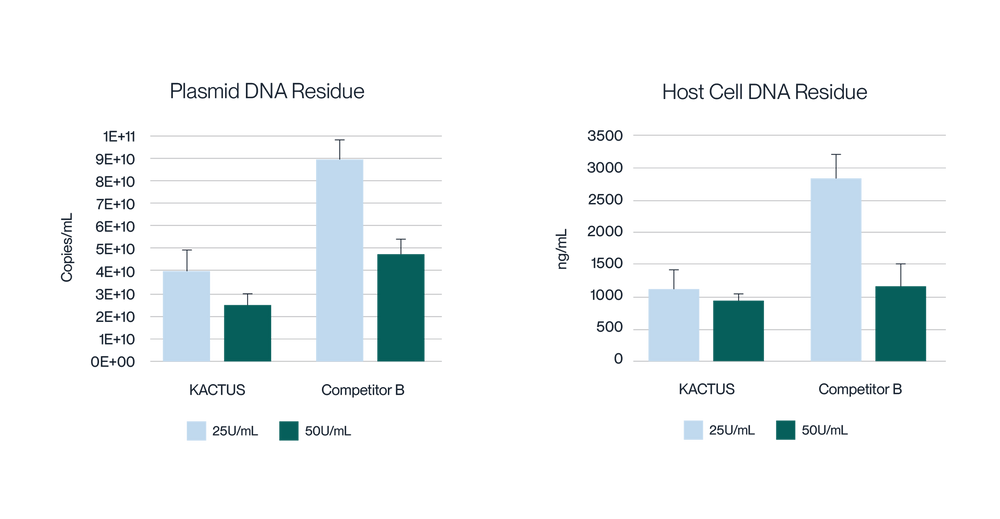
Virus harvest solution was treated with 25U/mL and 50U/mL endonuclease at 37°C for 2 hours, respectively. Detection of plasmid DNA (pDNA) residue (left) and host cell DNA (HCD) (right) residue was analyzed. KACTUS MaxNuclease has higher degradation activity versus Competitor B demonstrated by lower pDNA and HCD residue for both 25U/mL and 50U/mL working concentrations.

Customize your protein