The Role of Delta-like Ligand 3 (DLL3) in Targeted Therapy for Cancer
By Yujiao Zhang
On November 13, 2023, Legend Biotech officially announced that its wholly-owned subsidiary, Legend Biotech Ireland Limited, has entered into an exclusive license agreement with Novartis Pharma AG for the chimeric antigen receptor T-cell (CAR-T) cell therapy candidate LB2102 (NCT05680922) targeting Delta-like ligand protein 3 (DLL3) [1]. This licensing agreement grants Novartis the global exclusive rights to develop, manufacture, and commercialize LB2102, and allows Novartis to apply its T-Charge™ platform to the production. Legend Biotech will receive a $100 million upfront payment and potential milestone payments up to $1.01 billion. This agreement not only brings the classic target DLL3 back into focus but also demonstrates the great potential of CAR-T therapy for solid tumors such as small cell lung cancer (SCLC), which is highly prevalent but has lacked effective treatments.

Figure 1: LB2102 [2]
About DLL3
Neuroendocrine tumors, including SCLC, pose significant challenges in clinical treatment due to their high heterogeneity and complexity. DLL3 is a classic target for diseases like SCLC, and is involved in various biological functions. It is a single-pass transmembrane protein that acts as an inhibitory Notch ligand [3]. DLL3 is responsible for regulating the differentiation of neuroendocrine and epithelial cells during embryonic lung development. Its structure comprises a DLS domain in the extracellular region that is highly conserved in the ligand family, EGF-like repeats, and a short intracellular domain.

Figure 2: Schematic diagram of DLL3 structure [4, 5].
Unlike other mammalian Notch family members, DLL3 resides in the Golgi apparatus and cytoplasmic vesicles during normal development. It interacts with unprocessed full-length Notch1 and DLL1, preventing them from localizing on the cell surface [6]. However, in high-grade neuroendocrine tumors including SCLC, such as large cell neuroendocrine lung cancer (LCNEC), neuroendocrine carcinomas of certain other sites, and prostate cancer, DLL3 is highly upregulated and abnormally expressed on the cell surface, making it a potential therapeutic target.

Figure 3: DLL3 expression on the surface of tumor cells [7]
Dysregulation of Notch signaling caused by DLL3 plays a pro-carcinogenic role in many malignant tumors. However, it can inhibit tumor growth in gliomas, malignant gliomas, and primary liver cancers. This indicates that DLL3 plays different roles depending on the cellular environment of the tumor. Additionally, DLL3 is considered a transcriptional target of Achaete-scute homolog 1 (ASCL1), which is a key transcription factor for neurogenesis and differentiation [8], suggesting that DLL3 is involved in a wider range of physiological functions.
Drugs targeting DLL3
Drugs targeting DLL3 are focused on the development of antibody-drug conjugates (ADCs), bispecific or multispecific antibodies, and CAR-T therapies. Several promising drugs have emerged, such as Rovalpituzumab tesirine (Rova-T), an ADC developed by Abbvie. Rova-T is a first-of-its-kind antibody-drug conjugate targeting DLL3 that showed good results in preclinical and early clinical studies. However, two phase III studies (TAHOE and MERU trials) evaluating Rova-T as a second-line treatment and first-line maintenance therapy in advanced SCLC were terminated due to failure. Nevertheless, new breakthroughs in other combination therapies are expected.

Figure 4: Mechanism of action of drugs targeting DLL3 [9]
Both Tarlatamab developed by Amgen and HPN 328 developed by Harpoon have also attracted attention. Both are antibody drugs indicated for SCLC. Tarlatamab is a bispecific antibody developed based on BiTE technology targeting DLL3 and CD3, serving as a bridge between T cells and tumor cells to achieve targeted T-cell immunotherapy. HPN 328 is a trispecific antibody targeting CD3, ALB (albumin), and DLL3, with extended half-life and enhanced permeability.
|
Drug Name |
Type |
Company |
Clinical Trial Phase |
Clinical Trial ID |
|
Rovapituzumab tesirine |
ADC |
Abbvie |
Phase 3 |
NCT03061812 |
|
HPN 328 |
Tri-specific antibody |
Harpoon Therapeutics |
Phase 2 |
NCT04471727 |
|
Tarlatamab |
Bi-specific antibody |
Amgen |
Phase 3 |
NCT05740566 |
|
BI 764532 |
Bi-specific antibody |
Boehringer Ingelheim |
Phase 2 |
NCT05882058 |
|
QLS31904 |
Bi-specific antibody |
Qilu Pharmaceutical |
Phase 1 |
NCT05461287 |
|
AMG 119 |
CAR-T |
Amgen |
Phase 1 |
NCT03392064 |
|
LB 2102 |
CAR-T |
Legend Biotech USA Inc |
Phase 1 |
NCT05680922 |
Table 1: Examples of clinical drugs targeting DLL3
As a star target for SCLC, DLL3 continues to make new clinical progress. Moreover, a large number of basic research and clinical trials have also confirmed that DLL3 is closely related to various other tumors. KACTUS has developed high-quality DLL3 recombinant protein products to support drug development for diseases related to DLL3, looking forward to the future market launch of superior drugs to benefit patients.
Features of KACTUS DLL3 proteins
- Expressed in mammalian cells for natural protein modification.
- Provides DLL3 domain protein to aid in drug optimization.
- Covers multiple species including human, mouse, and monkey, to meet the needs of cross-species experiments.
- Various tag designs offer more research options.
- Verified biological activity for reliable quality assurance.
- Stable batch-to-batch consistency to save on drug development costs.
Product Validation Examples
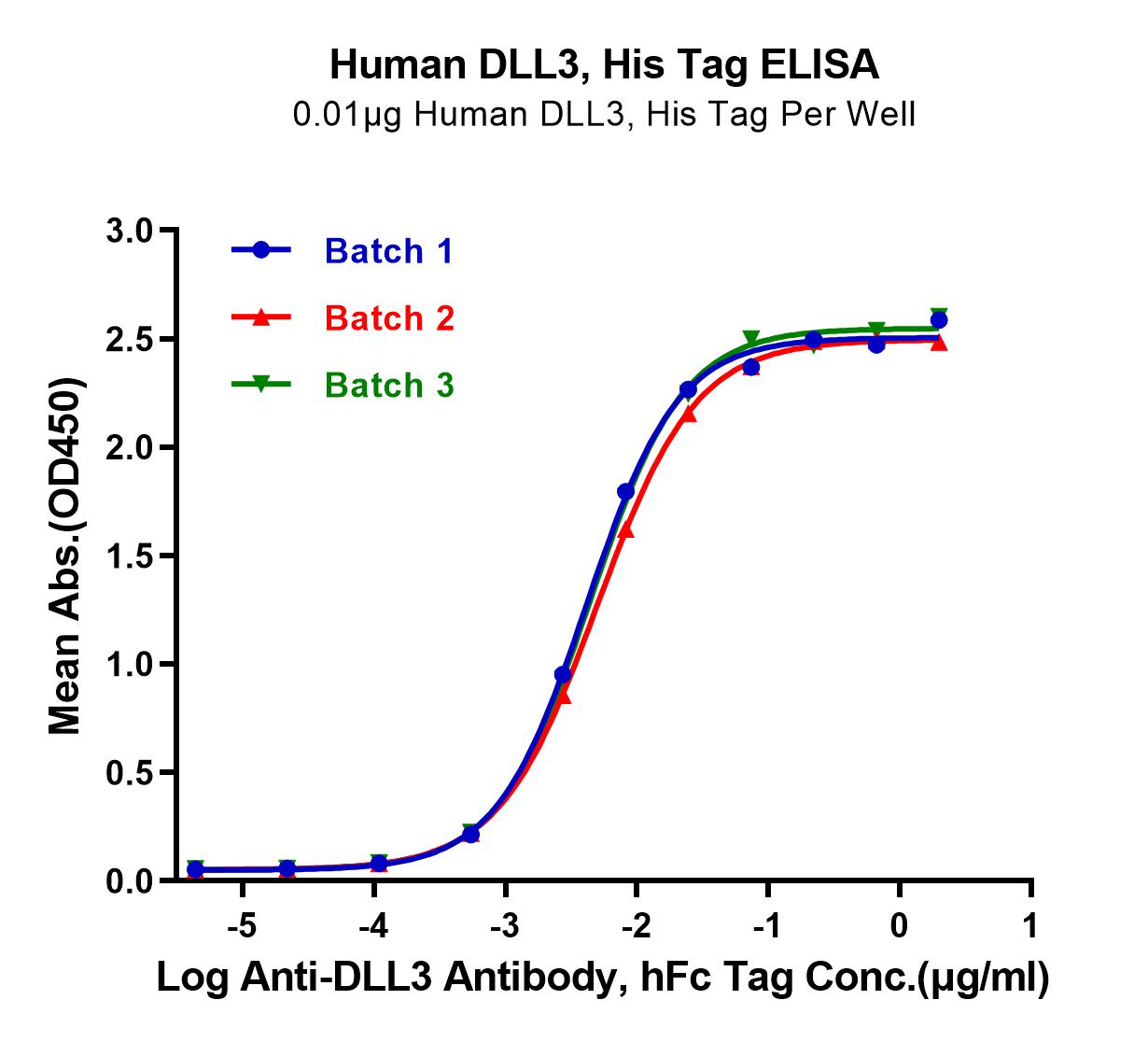
Figure 5: Verified by ELISA, Human DLL3 can bind to Anti-DLL3 Antibody and has good batch-to-batch stability.

Figure 6: Verified by ELISA, Cynomolgus DLL3 can bind to Anti-DLL3 Antibody, with an EC50 of 5.0 ng/ml.
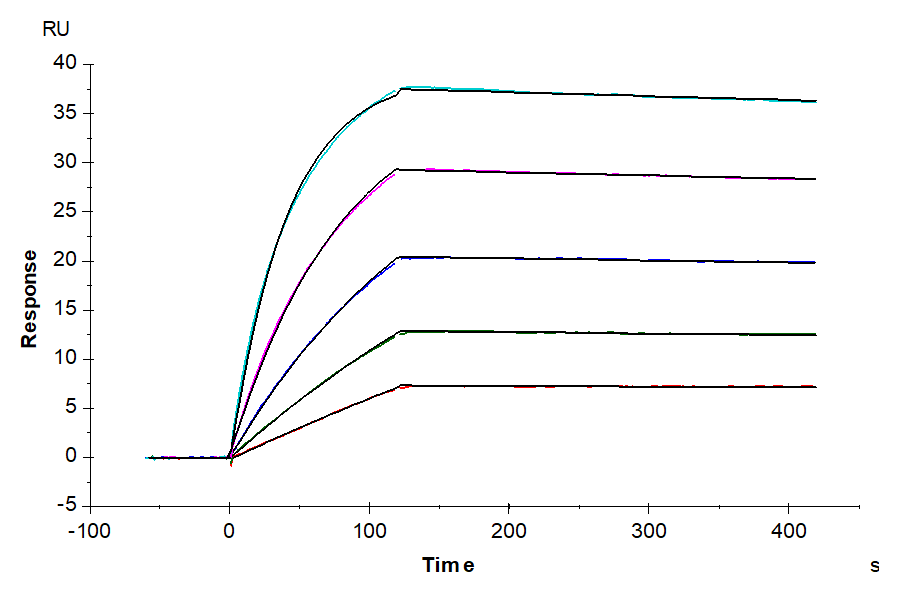
Figure 7: Human DLL3 can bind to Anti-DLL3 Antibody as detected by SPR experiment, with an affinity constant of 0.22 nM. Anti-DLL3 Antibody captured on CM5 Chip via Protein A can bind Human DLL3, His Tag with an affinity constant of 0.22 nM as determined in SPR assay (Biacore T200). This example highlights how binding interactions can be quantified through our surface plasmon resonance service offering precise kinetic profiles for protein-ligand interactions.
Available DLL3 Products
Expressed from HEK293 cells. Purity > 95%
|
Product Name |
Tag |
|
N-His |
|
|
N-His-Flag |
|
|
Human DLL3 Domain (311-479) Protein |
C-His-Avi |
| Biotinylated Human DLL3 Domain (311-479) Protein | C-His-Avi |
|
N-His |
|
|
C-His |
|
|
N-His |
|
Also check out our CD3 proteins here, including homodimers and heterodimers.
References
[1] https://legendbiotech.com/legend-news/legend-biotech-announces-exclusive-global-license-agreement-for-certain-car-t-therapies-targeting-dll3/
[2] https://investors.legendbiotech.com/static-files/3cb06824-4ee5-4610-951c-8a3bd8e9ec3b
[3] Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007 May;12(5):535-42. doi: 10.1634/theoncologist.12-5-535. PMID: 17522241.
[4] Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000 Apr;24(4):438-41. doi: 10.1038/74307. PMID: 10742114.
[5] https://www.uniprot.org/uniprotkb/Q9NYJ7/
[6] Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet. 2011 Mar 1;20(5):905-16. doi: 10.1093/hmg/ddq529. Epub 2010 Dec 7. PMID: 21147753.
[7] https://abbviescience.com/
[8] Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang A, Liu D, Lopez-Molina J, Milton M, Park A, Pysz MA, Shao H, Slingerland B, Torgov M, Williams SA, Foord O, Howard P, Jassem J, Badzio A, Czapiewski P, Harpole DH, Dowlati A, Massion PP, Travis WD, Pietanza MC, Poirier JT, Rudin CM, Stull RA, Dylla SJ. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015 Aug 26;7(302):302ra136. doi: 10.1126/scitranslmed.aac9459. PMID: 26311731; PMCID: PMC4934375.
[9] Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019 Jun 18;12(1):61. doi: 10.1186/s13045-019-0745-2. PMID: 31215500; PMCID: PMC6582566.