Engineered adenine base editor for gene knockout and correction
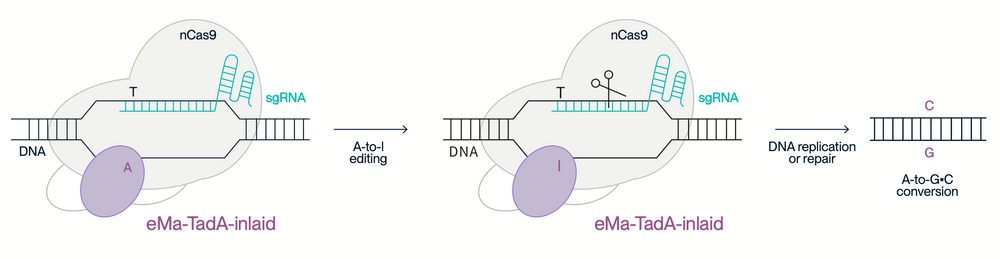
The supra-ABE enzyme is a high-performance adenine base editor featuring exceptional editing efficiency, a broad activity window, and low off-target effects—making it a powerful tool for precise, targeted cell and gene therapy applications. It is built on a patented architecture where the novel eMa-TadA adenine deaminase is chimerically embedded within a Cas9 nickase (Cas9n) to enhance editing precision and flexibility.
Developed exclusively by Lumiere Therapeutics, supra-ABE was further optimized for commercial production by KACTUS using our SAMS™ platform (Structure-Aided design and Multiplex Screening) to ensure high purity, superior stability, and consistent editing performance. KACTUS is the exclusive provider of this advanced base editing enzyme.
How does supra-ABE base editor work?
Efficient A-to-G Conversion with Wide Editing Window
The engineered adenine deaminase eMa-TadA in supra-ABE catalyzes the conversion of adenine (A) to inosine (I) in DNA. During replication, inosine is interpreted as guanine (G), enabling precise A-to-G substitutions within positions 4–14 of the editing window. This process occurs without the need for donor templates or the induction of double-strand DNA breaks, minimizing genomic disruption.
Applications of supra-ABE base editor
Gene Knockout
Gene knockout can be achieved by introducing A-to-G or T-to-C substitutions that disrupt key genetic elements—such as mutating the start codon (ATG) or altering canonical splice sites (GT-AG)—effectively disabling gene function.
Gene Correction
The supra-ABE editor enables precise single-base correction of pathogenic mutations. By converting specific adenine or thymine bases to guanine or cytosine, it restores normal gene function without introducing double-strand breaks.
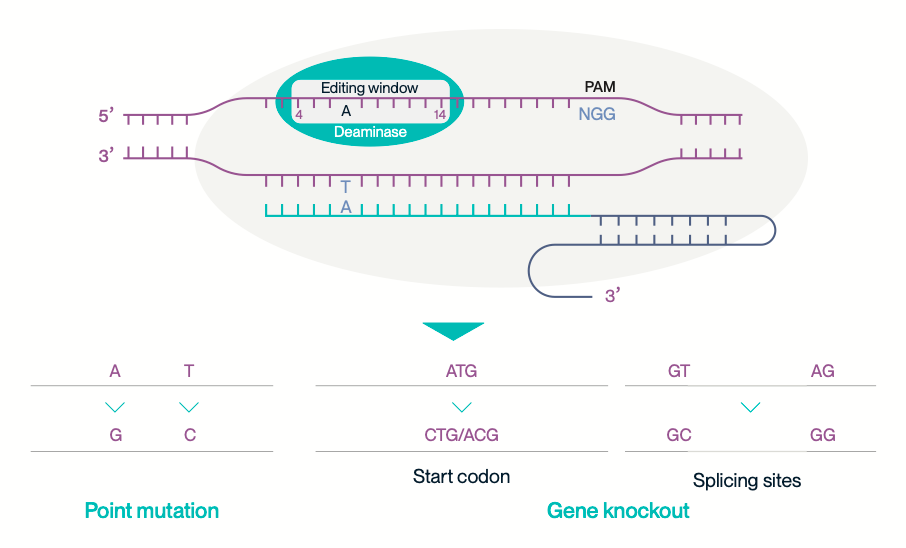
Gene knockout and gene correction applications of supra-ABE.
Product Performance Data
High Base Editing Activity Verus ABE8e
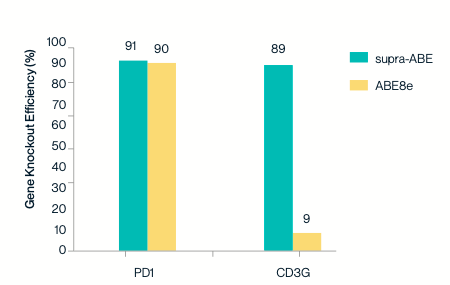
Sanger sequencing and EditR analysis demonstrate that supra-ABE efficiently mediates A-to-G conversion in the start codon (ATG) of PD1 and CD3G genes, disrupting translation initiation and achieving approximately 90% knockout efficiency.
Wide Editing Window
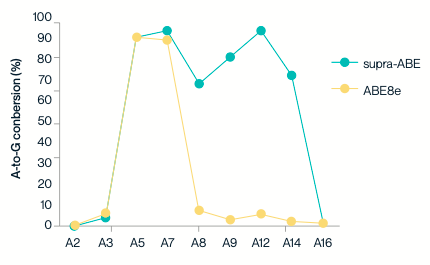
Sanger sequencing and EditR analysis results show that supra-ABE can convert A to G within the editing window, with varying editing efficiencies for adenine (A) bases at different positions—the highest editing efficiency exceeds 90%. Additionally, the editing window of supra-ABE is wider than that of ABE8e.
Efficient Knockout of CD3G Protein
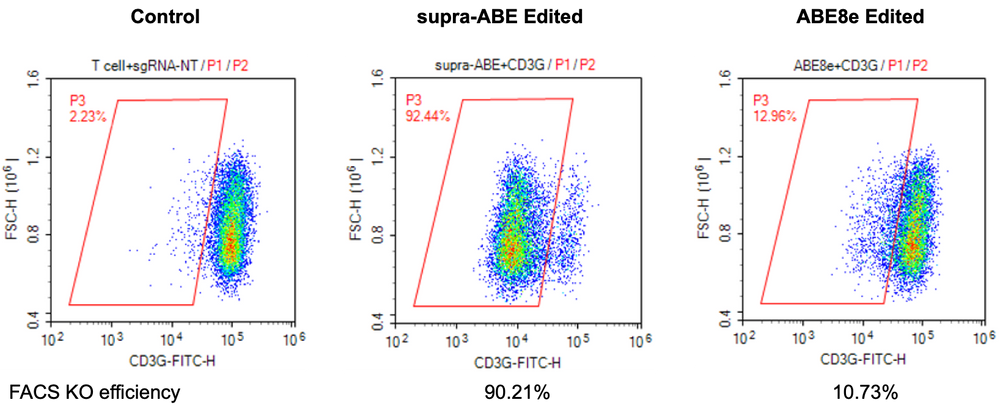
FACS analysis demonstrated that supra-ABE efficiently knocked out the CD3G protein, achieving a knockout efficiency of 90.21%.
Greater than 95% Purity
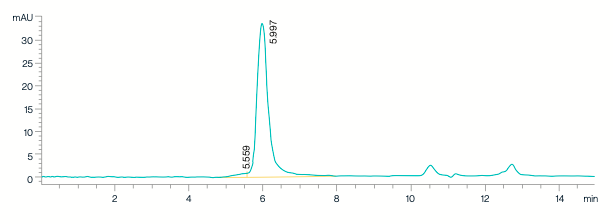
SEC-HPLC analysis confirms supra-ABE purity exceeds 95.0%.
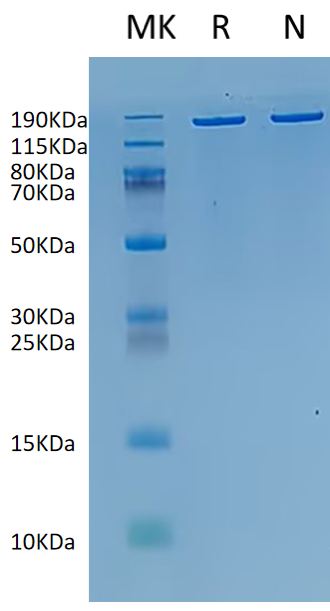
Bis-Tris PAGE analysis confirms supra-ABE purity exceeds 95.0%.
Advantages of supra-ABE

Proprietary Intellectual Property
Supra-ABE is a commercial-grade adenine base editor developed under an independent patent, ensuring freedom to operate and strong intellectual property protection.
High Editing Efficiency
Engineered for robust performance, supra-ABE enables efficient editing across a wide range of gene loci, supporting high-throughput target screening.

Broad Editing Window
With an expanded editing window (positions 4–14), supra-ABE outperforms traditional ABE8e, offering greater flexibility in target site selection.
Flexible Targeting Abilities
Ideal for gene therapy, cell therapy, and epigenetic applications, supra-ABE enables efficient editing of key regulatory elements—such as start codons (ATG) and splice sites (GT-AG)—supporting gene knockout, exon skipping, and more.
Product Specifications & Ordering
Quality Control Criteria
| Parameter | Specification |
|---|---|
| Concentration | 9.0-11.0mg/mL |
| Purity (Bis-Tris PAGE) | ≥ 80.0% |
| Purity (SEC-HPLC) | ≥ 80.0% |
| Endotoxin | ≤ 10.0EU/mg |
Ordering Information
Catalog No. CAS-EE145
Product Name: supra-ABE
Available Sizes: 100UG / 1MG
To request a quote or speak with a representative, please contact us at support@kactusbio.us.
Explore supra-ABE Products & Resources
Product Brochure
Access detailed information on supra-ABE to see how it may support your research.
Related Products & Information
Base Editing News & Insights
NK Cell Therapy Gets IND FDA Clearance with AccuBase® Base Editor from KACTUS
AccuBase™ Base Editor Continues to Advance NK Cell Therapies
Base Editing Technology Leads a New Chapter in Drug Development
Global Release of the First Commercial Base Editor on the Market: AccuBase™
Gene Editing News & Insights
KACTUS' Cas9 Enzyme Powers IND-Approved Cell Therapy
Panorama of CRISPR Gene Editing Clinical Applications: 2024 Review
2024 Milestones in CRISPR Clinical Pipelines
IND Approval for Solid Tumor Therapy Using KACTUS Cas9 Enzyme
Gene Editing Drugs from the EMA Review Perspective
FDA New Guidelines Pave the Way for Gene Editing Drug Development