supra-ABE (Adenine Base Editor) Summary
Product Description |
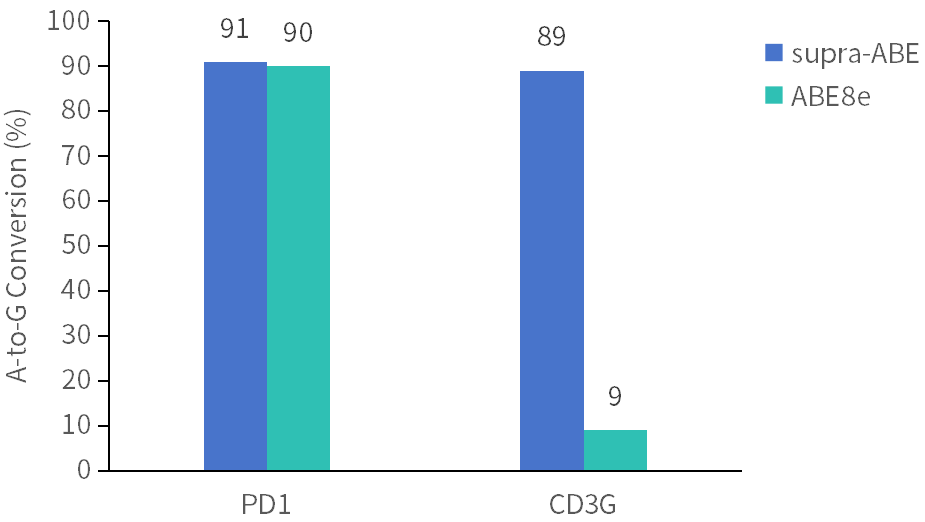
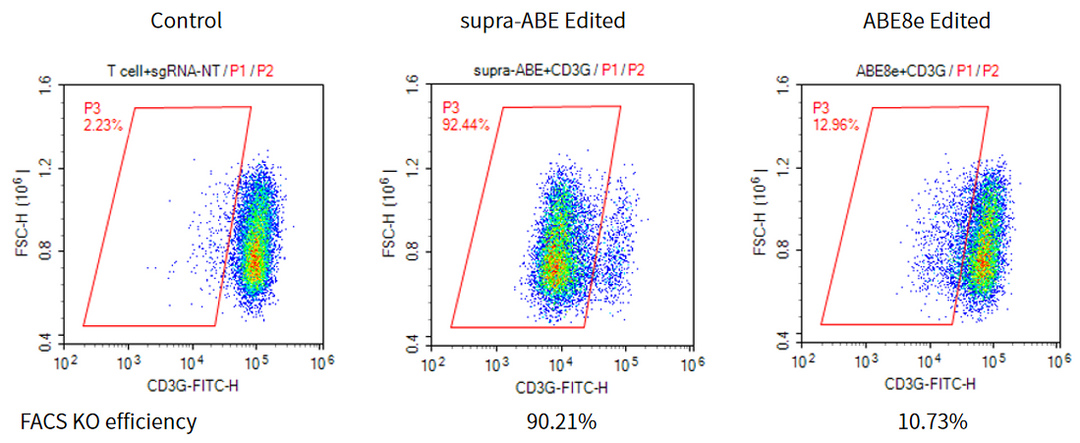
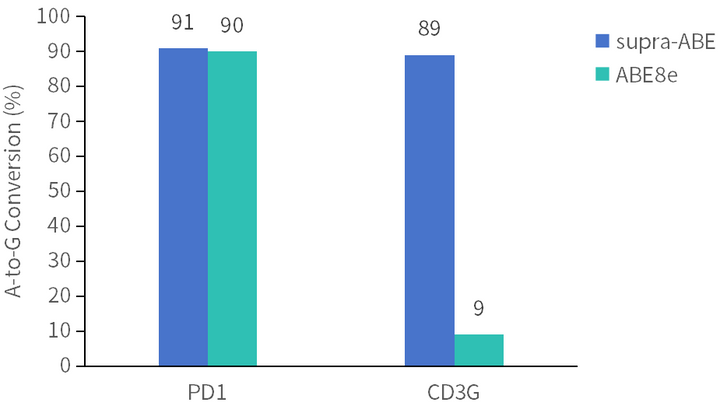
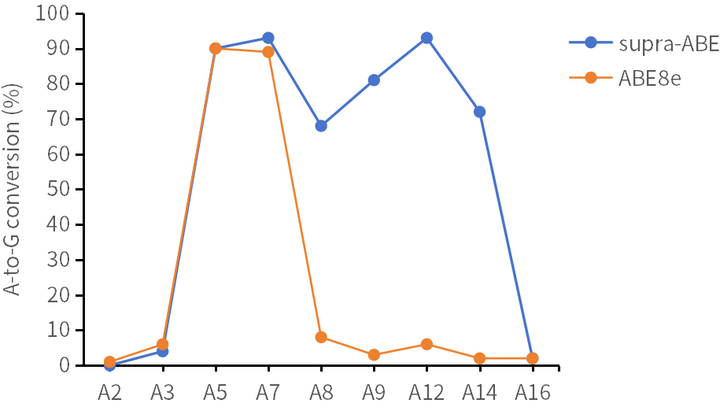
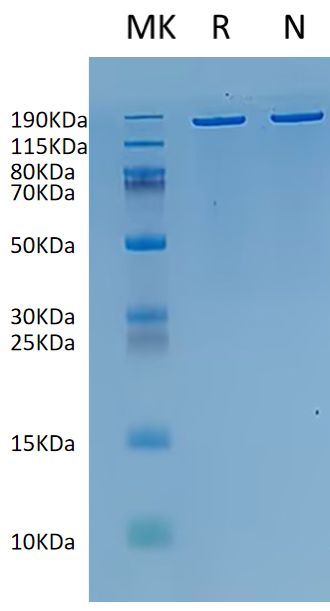
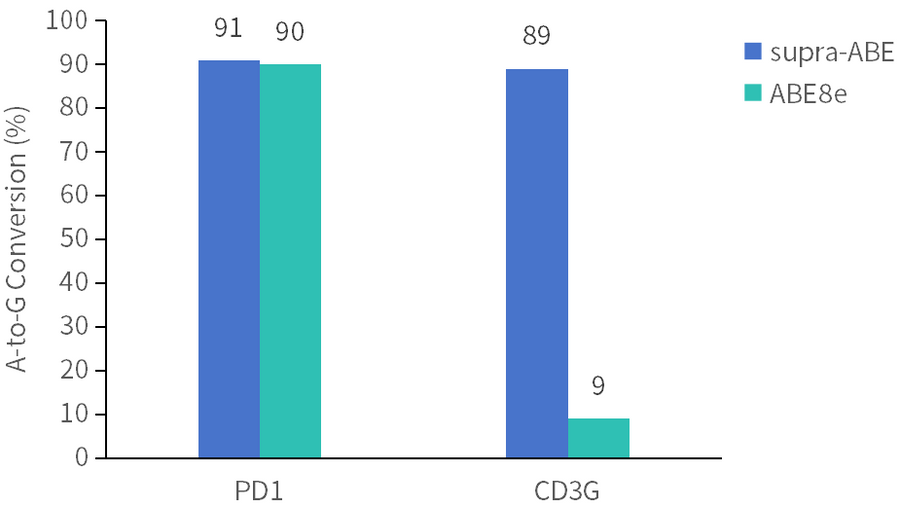
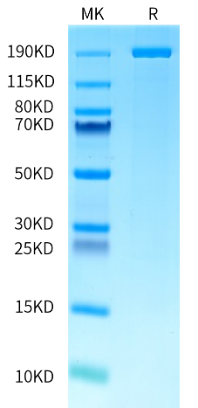
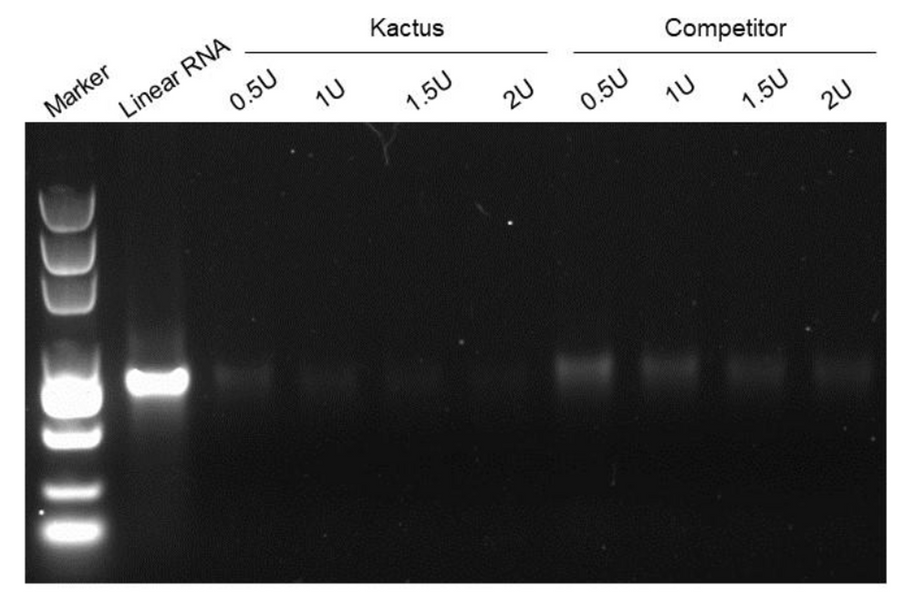
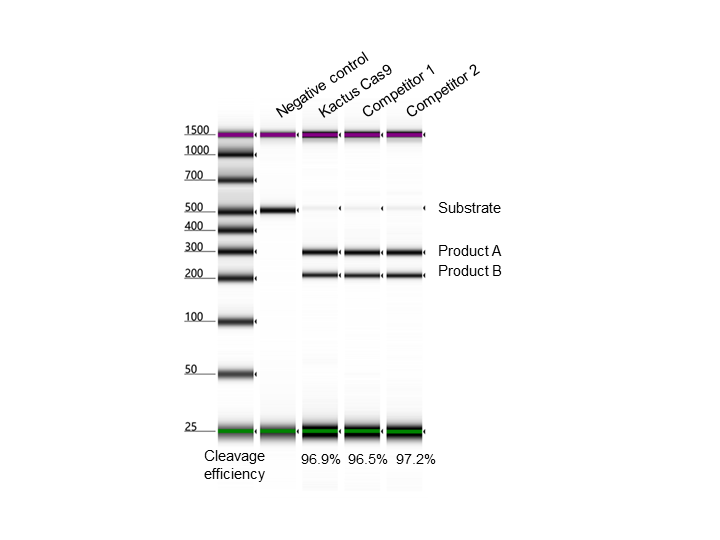
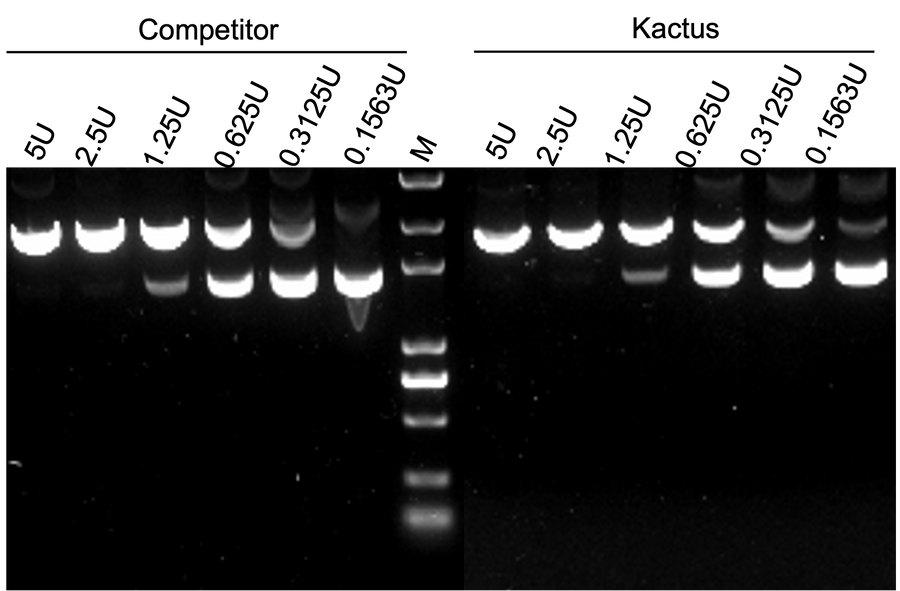
supra-ABE is an adenine base editor comprising the exclusively patented adenine deaminase eMa-TadA fused with Cas9 nickase (Cas9n), where eMa-TadA is embedded within Cas9n. The eMa-TadA deaminase catalyzes the deamination of adenine in DNA, converting it to inosine. During DNA replication, inosine is recognized as guanine, enabling A-to-G base substitution within editing window positions 4-14 without requiring a donor template or inducing DNA double-strand breaks. The supra-ABE system demonstrates high editing activity, broad effective editing windows, and low off-target rates, making it a versatile tool for applications in cell and gene therapy. |
Product Background |
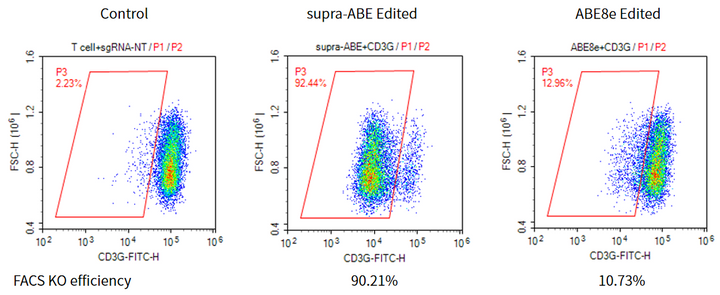
Applications 1. Gene Knockout: Gene knockout is achieved by mutating the start codon ATG or altering the canonical splice sites GT-AG through A-to-G or T-to-C substitutions, thereby disrupting gene function. 2. Gene Correction: Precise repair of pathogenic single-base mutations (A-to-G or T-to-C substitutions at specific adenine or thymine sites) enables restoration of normal gene function through single-base editing. |
supra-ABE (Adenine Base Editor) Specifications/Details
Concentration |
10 mg/mL |
Source |
E. coli |
Molecular Weight |
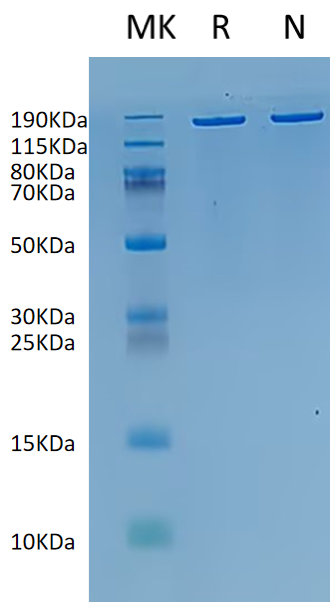
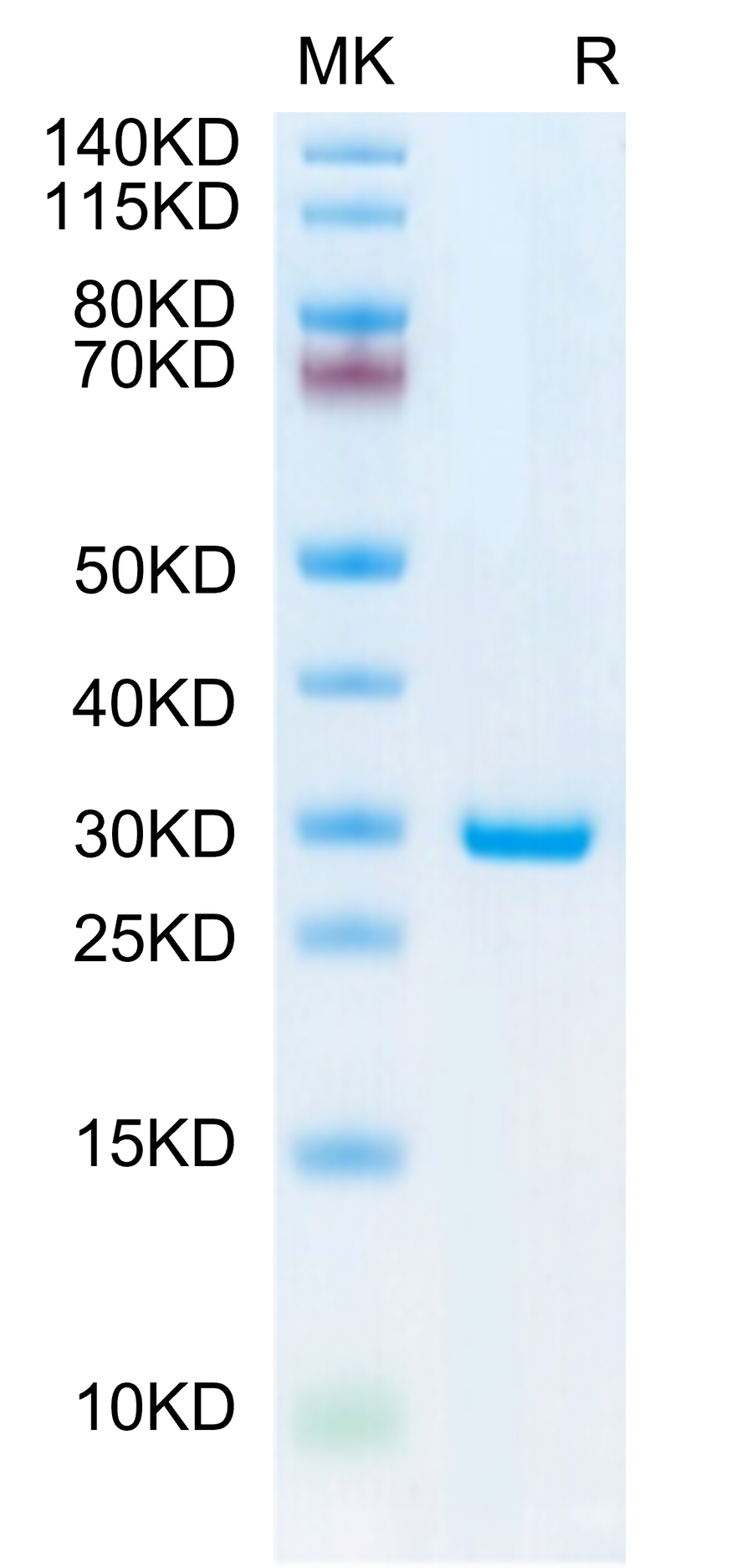
183.83 kDa |
Purity |
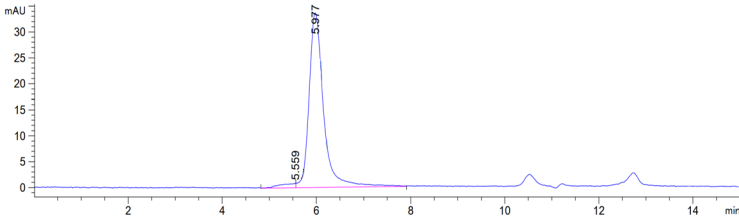
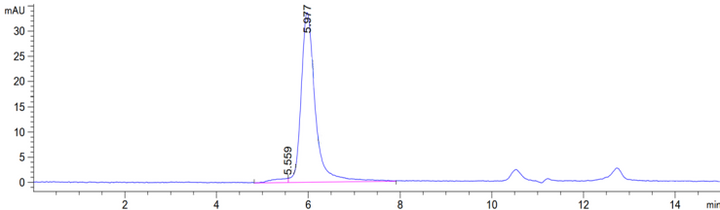
≥ 80% |
Endotoxin |
≤ 10.0 EU/mg |
Quality Standards |
Concentration: 9.0-11.0mg/mL Purity (Bis-Tris PAGE): ≥ 80.0% Purity (SEC-HPLC): ≥ 80.0% Endotoxin: ≤ 10.0EU/mg |
Shipping, Reconstitution, & Storage
Form |
Liquid |
Formulation |
30mM Tris, 300mM NaCl, 50% Glycerol, 0.1mM EDTA, 5mM DTT, pH 7.8 |
Stability And Storage |
Transport on dry ice. Store at -80 ±10°C. Avoid repeated freezing and thawing. |
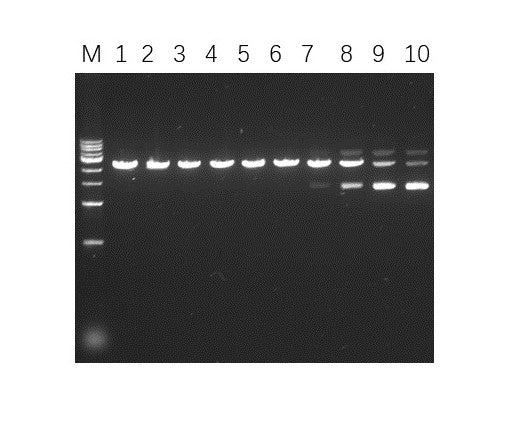
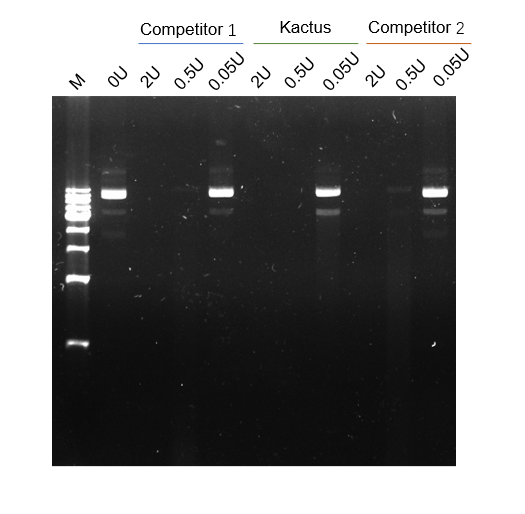
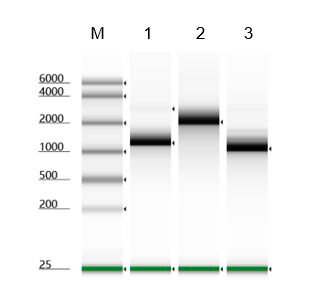
Validation Images
Related Products
Have more questions?
Check out our Frequently Asked Questions page for more details about product specifications, ordering, and shipments.
Contact Us
Recently viewed