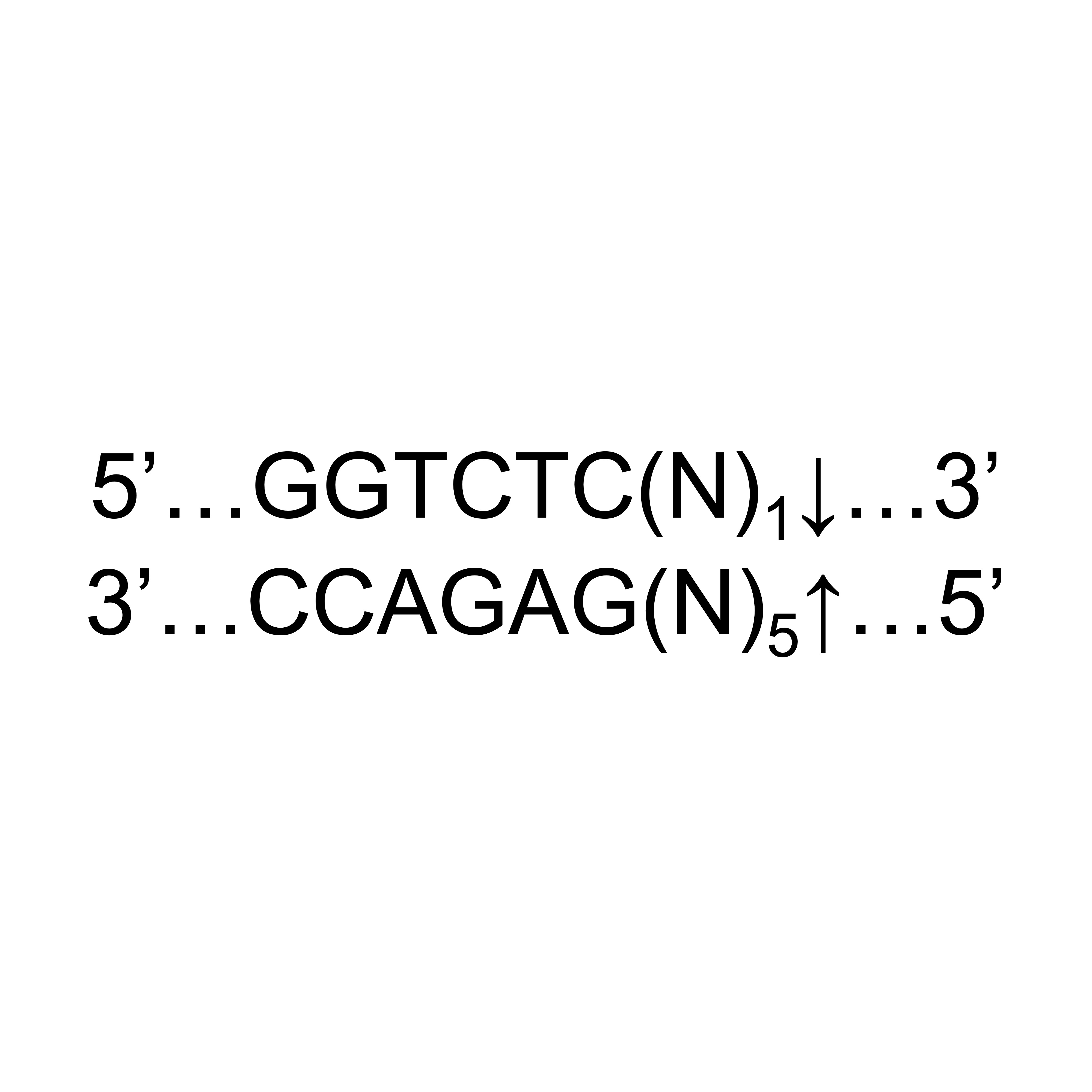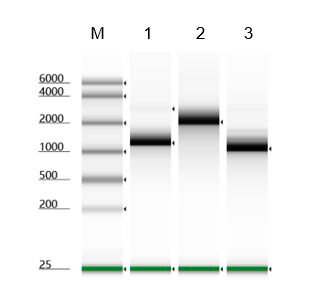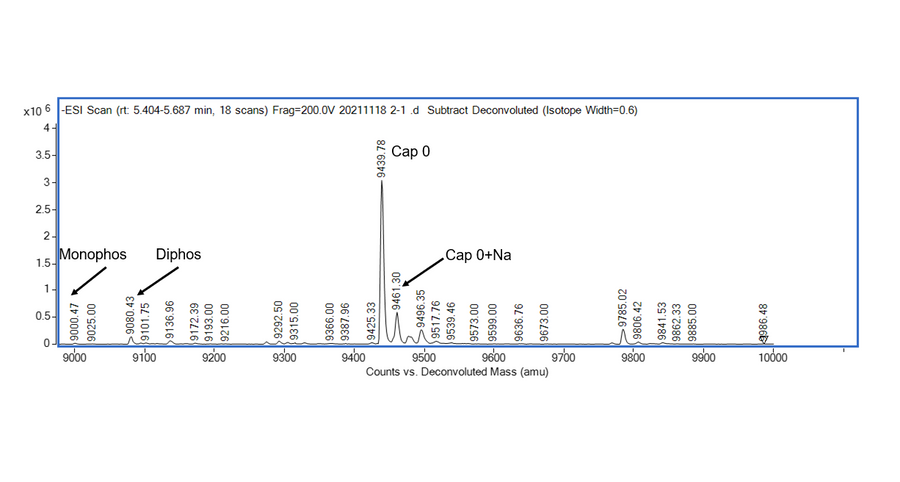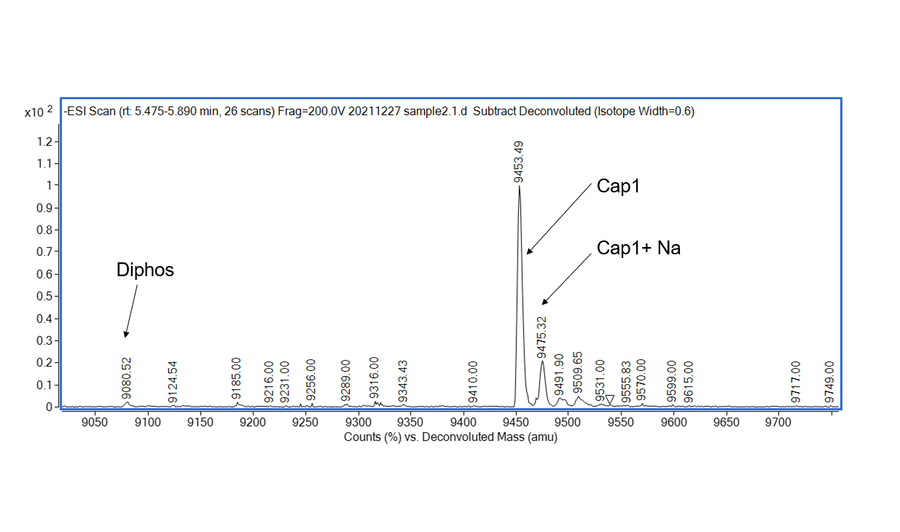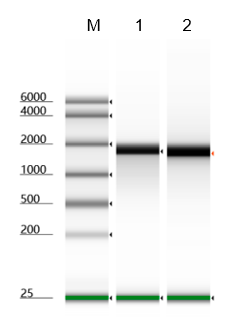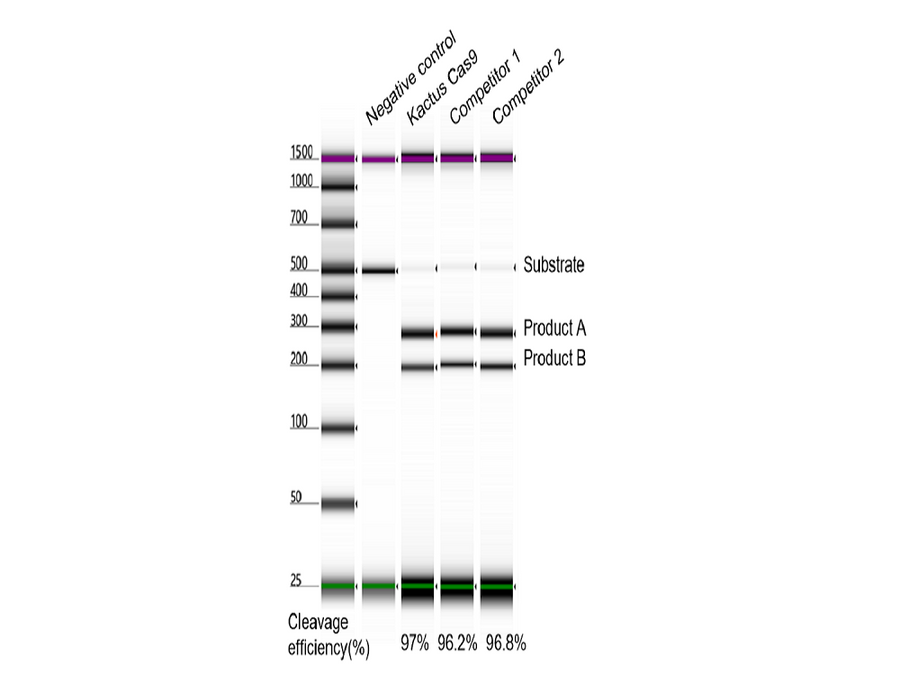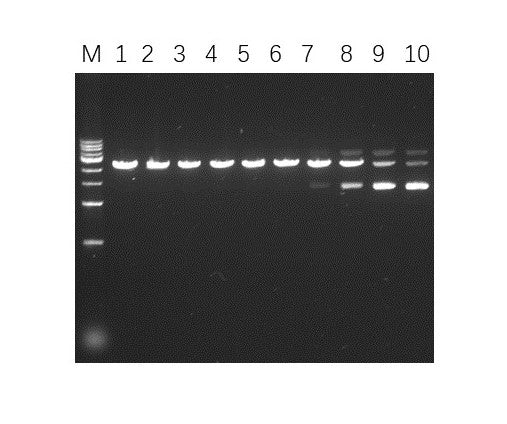
- Description: Recognizes asymmetric DNA sequences and cleaves outside of their recognition sequence
- Applications: Genotyping
SNP
Plasmid linearization - Quality: FDA DMF #037503
Special formulation enables BSA-free reaction
Manufactured according to GMP guidelines
Antibiotic-free and animal-free production