Product Description |
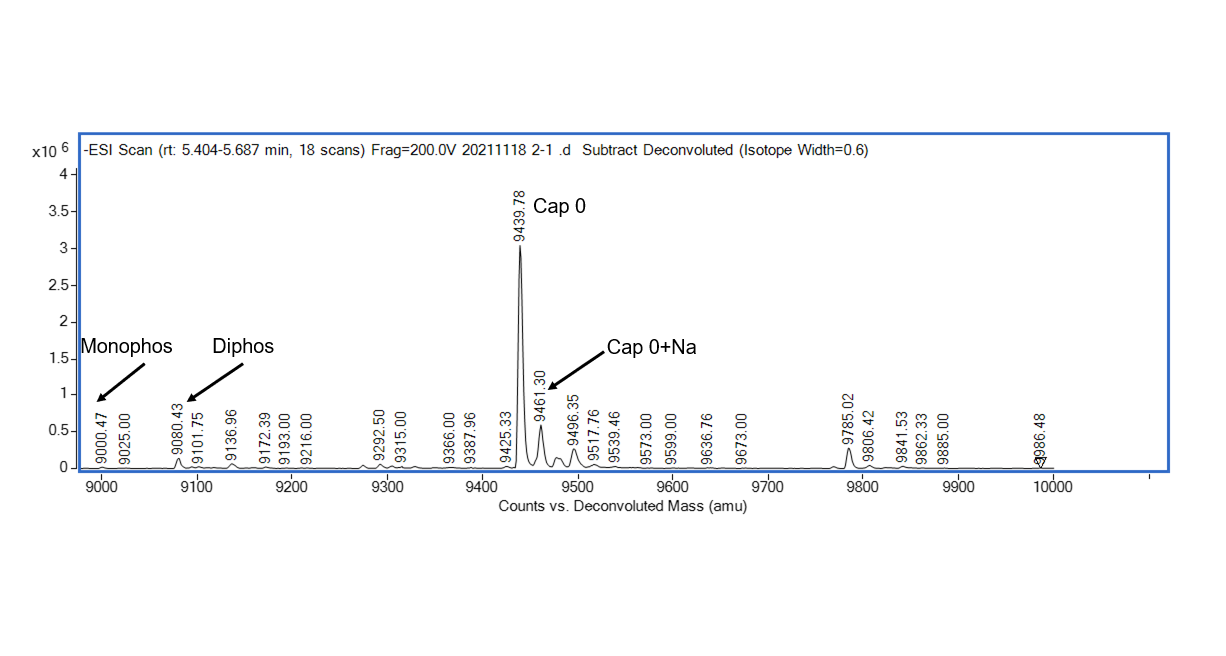
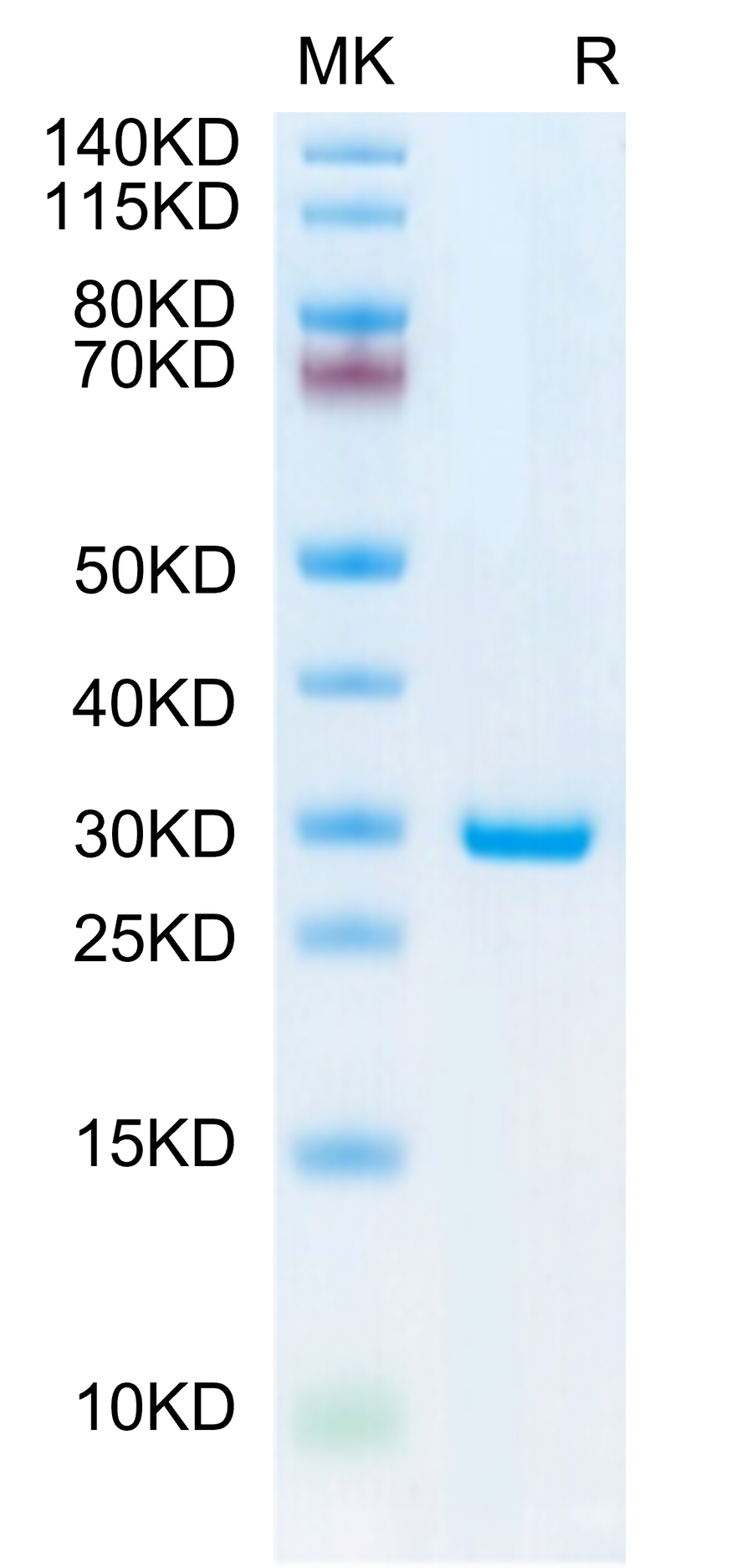
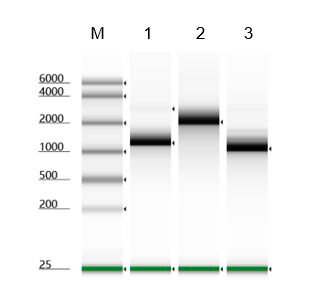
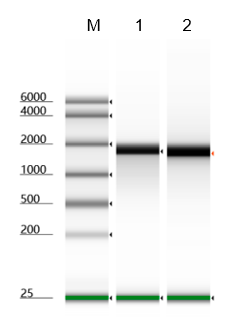
The 7-methylguanosine cap structure (m7Gppp, Cap0) is a specific feature in eukaryotic mRNA, which is required for mRNA stability, splicing, nuclear export, effective translation, and mRNA decay. Vaccinia Capping Enzyme, a heterodimer of D1 and D12 subunits, adding the Cap0 structure to the 5' end of the RNA. The D1 subunit comprises an N-terminal RNA triphosphatase (TPase), a guanylyltransferase (GTase), and a C-terminal guanine-N7-methyltransferase (MTase). The D12 subunit binds and stimulates the MTase.
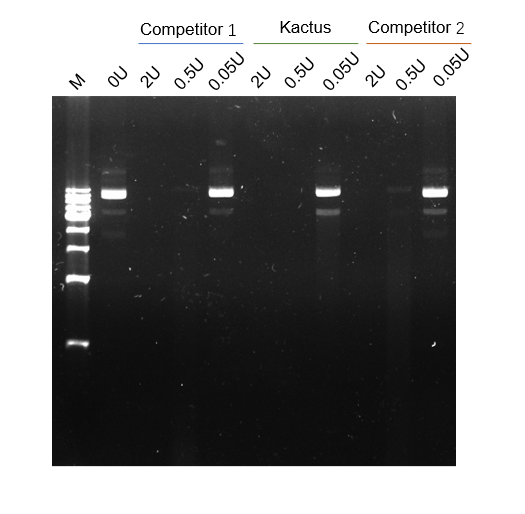
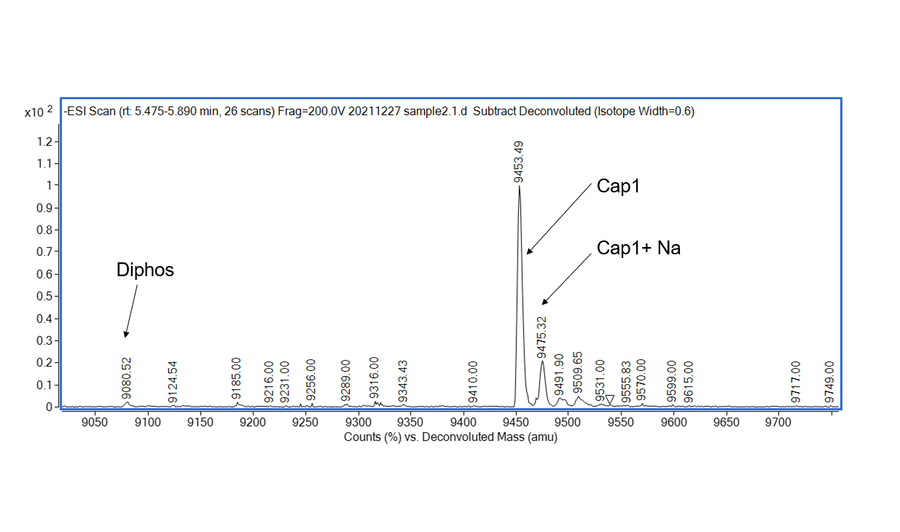
NOTE: mRNA Cap 2'-O-Methyltransferase (Kactus #GMP-MEH-VE101) is required to generate Cap 1 structure that reduces cellular innate immune response when the RNA is used in vivo .
This product has been filed with the FDA Drug Master Files (DMF) and is assigned DMF #038028. |