Immunotherapy Target: CD19 Antigen
By Mallory Griffin
CD19 remains a hot target in immunotherapy, driving innovation across a range of diseases, supported by advances in recombinant proteins for drug discovery. This is evidenced by recent approvals for new treatments in multiple sclerosis and leukemia and a major acquisition in the bispecific antibody space:
September 9, 2024: Zenas acquired clinical trial approval from the Center for Drug Evaluation (CDE) of the China National Medical Products Administration for Obexelimab injection, a new class 1 drug from Xencor, to treat adult relapsing multiple sclerosis (RMS).
August 9, 2024: Merck, through a subsidiary, acquired Tongren Biopharma’s CD3×CD19 bispecific antibody CN201 pipeline for $1.3 billion.
February 28, 2024: The CDE approved AstraZeneca’s CD19 bispecific antibody AZD0486 for treating relapsed or refractory B-cell acute lymphoblastic leukemia (r/r B-ALL).
Cluster of Differentiation 19 (CD19)
B-lymphocyte antigen CD19, also known as Cluster of Differentiation 19 (CD19), is a membrane protein expressed on all B cell lineages, malignant B cells, and follicular dendritic cells. However, hematopoietic stem cells, plasma cells, T cells, or other tissues, do not express CD19, which gives it a unique expression profile. The extracellular domain of CD19 has two C2-type Ig-like domains, while the intracellular C-terminal domain has multiple tyrosine residues, with Y391, Y482, and Y513 being essential to its biological function (Figure 1).

Figure 1. CD19 structure [1].
CD19 plays a crucial role in B cell proliferation, differentiation, activation, and antibody production. It forms a signal transduction complex with CD21, CD81, and CD225, regulating both B Cell Receptor (BCR)-dependent and -independent signaling, which drives autoantibody production and immune-mediated damage (Figure 2).

Figure 2. B Cell Receptor (BCR) signaling pathways [2].
CD19 Antibody Drugs
CD19’s expression in most B-cell malignancies makes it an ideal target for lymphoma treatments. Four antibody drugs targeting CD19 are currently on the market: one bispecific antibody (Blinatumomab), two monoclonal antibodies (Inebilizumab, Tafasitamab), and one ADC (Loncastuximab Tesirine), with more entering clinical trials. Common indications include diffuse large B-cell lymphoma (DLBCL), acute lymphoblastic leukemia (ALL), B-cell lymphoma, and follicular lymphoma (FL), with recent expansion into autoimmune inflammatory diseases.
Monoclonal Antibodies
The monoclonal antibody Obexelimab, developed by Xencor, uses an IgG4 design to bind both CD19 and FcγRIIb, inhibiting B cell function rather than depleting cells. This reduces side effects associated with cell lysis, with lower immunogenicity and a longer half-life. In November 2021, Zenas obtained global rights to Obexelimab from Xencor and plans to advance clinical research in IgG4-related diseases (IgG4-RD) and other autoimmune diseases. In September 2023, Zenas entered a strategic licensing and collaboration agreement with BMS, granting exclusive rights in Japan, South Korea, Taiwan, Singapore, Hong Kong, and Australia. Obexelimab is currently in phase 3 trials for warm autoimmune hemolytic anemia and IgG4-RD, highlighting CD19’s value in autoimmune disease.

Figure 3. Obexelimab mechanism of action [3].
Bispecific Antibodies
AstraZeneca’s AZD0486, featuring a low-affinity anti-CD3 fragment and a silenced Fc, minimizes cytokine release and prevents non-specific toxicity. Clinical trials show an ORR of 81.2% in B-cell non-Hodgkin lymphoma (B-NHL) and 75% in DLBCL. On the other hand, Roche’s Englumafusp alfa, takes a different approach by simultaneously targeting CD19 and 4-1BB, triggering potent co-stimulation to sustain immune cell activity and enhance efficacy.

Figure 4. Structure of Englumafusp alfa [4].
Antibody-Drug Conjugates (ADC)
In addition to the marketed Loncastuximab Tesirine (Zynlonta), several Antibody-Drug Conjugates (ADCs) are under clinical investigation, including Sanofi and ImmunoGen’s Coltuximab ravtansine, Seagen’s Denintuzumab mafodotin, and AbbVie’s ABBV-319, each with unique payload and linker designs.
Summary of Antibody Drugs Targeting CD19
| Company | Drug Name |
Target or conjugate |
Stage | Indications |
| Monoclonal Antibodies (mAb) | ||||
| Viela Bio / Horizon Therapeutics / Amgen | Inebilizumab | CD19 | Approved June 2020 | Neuromyelitis optica spectrum disorder |
| Incyte | Tafasitamab-cxix | CD19 | Approved July 2020 | Relapsed or refractory diffuse large B-cell lymphoma |
|
Zenas / Xencor / BMS
|
Obexelimab
|
CD19 and Fc gamma receptor IIb
|
Phase 3
|
Warm autoimmune hemolytic anemia |
| IgG4-related disease | ||||
|
BMS
|
MDX-1342
|
CD19
|
Discountinued after Phase I
|
Chronic lymphocytic leukemia |
| Rheumatoid arthritis | ||||
| IASO Biotherapeutics | IASO-782 | CD19 | Phase 1 | Autoimmune thrombocytopenia, warm autoimmune hemolytic anemia |
| Bispecific Antibodies (bsAb) | ||||
| Amgen | Blinatumomab | CD19 / CD3 | Approved December 2014 | Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia, indolent B-cell lymphoma |
| TeneoTwo / AstraZeneca | AZD0486 | CD19 / CD3 | Phase 3 | Untreated follicular lymphoma |
| Curon Biopharmaceutical / Merck | CN201 | CD19 / CD3 | Phase 1b / 2 | Acute lymphoblastic leukemia |
| Roche | Englumafusp alfa | CD19 / 4-1BB | Phase 1 / 2 | Relapsed or refractory non-Hodgkin's lymphoma |
| Lüzhu Biotechnology | K193 | CD19 / CD3 | Phase 1 | Advanced solid tumors |
| ITabMed |
A319
|
CD19 / CD3
|
Phase 1
|
Systemic lupus erythematosus |
| EVIVE Biotechnology | Relapsed or refractory B-cell lymphoma | |||
| Antibody Drug Conjugates (ADC) | ||||
| ADC Therapeutics | Loncastuximab Tesirine | CD19 with PBD conjugate | Approved April 2021 | Relapsed or refractory diffuse large B-cell lymphoma |
|
Sanofi / ImmunoGen
|
Coltuximab ravtansine
|
CD19 with DM4 conjugate
|
Discountinued after Phase 2
|
Diffuse large B-cell lymphoma
|
|
Seagen
|
Denintuzumab mafodotin
|
CD19 with MMAF conjugate
|
Discountinued after Phase 2
|
Diffuse large B-cell lymphoma, grade 3b follicular lymphoma, transformed lymphoma |
| B-cell lymphoma, diffuse large B-cell lymphoma, grade 3b follicular lymphoma | ||||
| Iksuda Therapeutics / LegoChem Biosciences | LCB73 | CD19 with PBD conjugate | Phase 1 | B-cell lymphoma, B-cell non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma |
| AbbVie | ABBV-319 | CD19 with Bromoacetamide conjugate | Phase 1 | Chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma |
| Fusion Proteins | ||||
| GT Biopharma | DT2219ARL | CD19 / CD22 / DT390 | Discontinued after Phase 1/2 | Refractory B-lineage leukemia, Refractory B-lineage lymphoma, Relapsed B-lineage leukemia, Relapsed B-lineage lymphoma |
Table 1. Antibody drugs targeting CD19 approved or in clinical trial.
CD19 as a CAR-T Therapy Target
CD19-Targeting CAR-T Therapy for Hematologic Malignancies
CD19’s unique expression on B cell lineages makes it a prime target for chimeric antigen receptor-T cell therapies (CAR-T), which have shown groundbreaking success in treating B-cell malignancies like diffuse large B-cell lymphoma (DLBCL) and acute lymphoblastic leukemia (ALL). CAR-T therapies, such as Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel), specifically target CD19 on malignant B cells, redirecting the patient’s immune system to destroy these cancerous cells. Their success has catalyzed further developments in CAR-T therapies targeting CD19 and similar antigens, offering new hope for patients with relapsed or refractory blood cancers.
With the rapid advancements in CAR-T and other cellular therapies, CD19’s role in immunotherapy continues to expand. Clinical studies have demonstrated that targeting CD19 effectively induces remission in a substantial portion of patients with otherwise untreatable B-cell cancers. This success underscores CD19's enduring relevance in hematologic oncology and its role in driving forward the next generation of immunotherapies.
CD19-Targeting CAR-T Therapy for Autoimmune Disease
CD19-targeting CAR-T cell therapies are emerging as promising treatments for autoimmune diseases like systemic lupus erythematosus (SLE) and antisynthetase syndrome (ASS), providing new options for patients unresponsive to traditional immunosuppressive therapies. In SLE, a chronic disease marked by immune attacks on healthy tissues, CD19 CAR-T cell therapy has demonstrated rapid remission and enhanced quality of life in cases resistant to conventional treatments [5]. Similarly, a case study on ASS, which involves muscle inflammation and interstitial lung disease, reported complete clinical remission in a patient after CD19 CAR-T therapy, highlighting its potential to manage refractory cases [6]. These findings underscore the expanding applications of CD19 CAR-T therapies beyond cancer treatment, offering hope for individuals with challenging autoimmune disorders.
High Quality CD19 Proteins from KACTUS Available Off-the-Shelf
CD19 is advancing not only in hematologic cancers but also in autoimmune diseases, demonstrating significant therapeutic potential and market value. To support CD19-targeted drug development, KACTUS provides high-quality recombinant CD19 proteins. These proteins, expressed in mammalian cells, include multiple species and tag configurations, undergo rigorous quality testing, and are suitable for various applications such as animal immunization, antibody screening, and drug functionality validation.
Product Validation Data

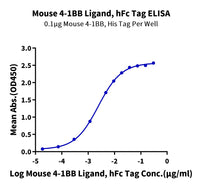
Figure 5. Immobilized Human CD19, His Tag at 2 μg/ml (100 μl/Well) on the plate. Dose response curve for Coltuximab, hFc Tag with the EC50 of 5.3 ng/ml determined by ELISA.

Figure 6. Immobilized Human CD19, His Tag at 1 μg/ml (100 μl/well) on the plate. Dose response curve for Denintuzumab, hFc Tag with the EC50 of 2.1 ng/ml determined by ELISA.

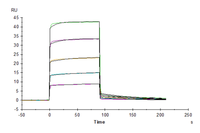
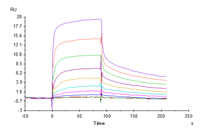
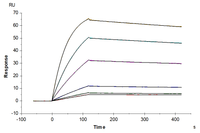
Anti-CD19 Antibody, hFc Tag captured on CM5 Chip via Protein A can bind Cynomolgus/Rhesus macaque CD19, His Tag with an affinity constant of 0.46 μM as determined in SPR assay.
CD19 Proteins Product List
|
Catalog No. |
Product Description |
Tag |
Expression System |
|
His Tag |
HEK293 |
||
|
His-Avi Tag |
HEK293 |
||
|
His Tag |
HEK293 |
||
|
His Tag |
HEK293 |
||
|
CD1-HM119F |
FITC-compatible Human CD19 |
His Tag |
HEK293 |
References
[1] Tedder, T. F. (2009). CD19: A promising B cell target for rheumatoid arthritis. Nature Reviews Rheumatology, 5(10), 572-577. https://doi.org/10.1038/nrrheum.2009.184
[2] Aribi, M. (2020). Introductory Chapter: B-Cells. IntechOpen. doi: 10.5772/intechopen.90636
[3] https://investors.xencor.com/static-files/e704e415-b58a-430a-bfdb-8a04795178e9
[4] Tapia-Galisteo A, Álvarez-Vallina L, Sanz L. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. J Hematol Oncol. 2023 Jul 27;16(1):83. doi: 10.1186/s13045-023-01482-w.
[5] Rangel-Peláez, C., Martínez-Gutiérrez, L., Tristán-Manzano, M., Callejas, J. L., Ortego-Centeno, N., Martín, F., & Martín, J. (2024). CD19 CAR-T cell therapy: A new dawn for autoimmune rheumatic diseases? Frontiers in Immunology, 15, 1502712. https://doi.org/10.3389/fimmu.2024.1502712
[6] Taubmann, J., Knitza, J., Müller, F., Völkl, S., Aigner, M., Kleyer, A., Gary, R., Kretschmann, S., Boeltz, S., Atzinger, A., Kuwert, T., Roemer, F., Uder, M., Mackensen, A., & Schett, G. (2023). Rescue therapy of antisynthetase syndrome with CD19-targeted CAR-T cells after failure of several B-cell depleting antibodies. Rheumatology (Oxford, England), 63(1), e12. https://doi.org/10.1093/rheumatology/kead330