mRNA Enzymes → Learn more about our mRNA Enzymes
14 products
14 products
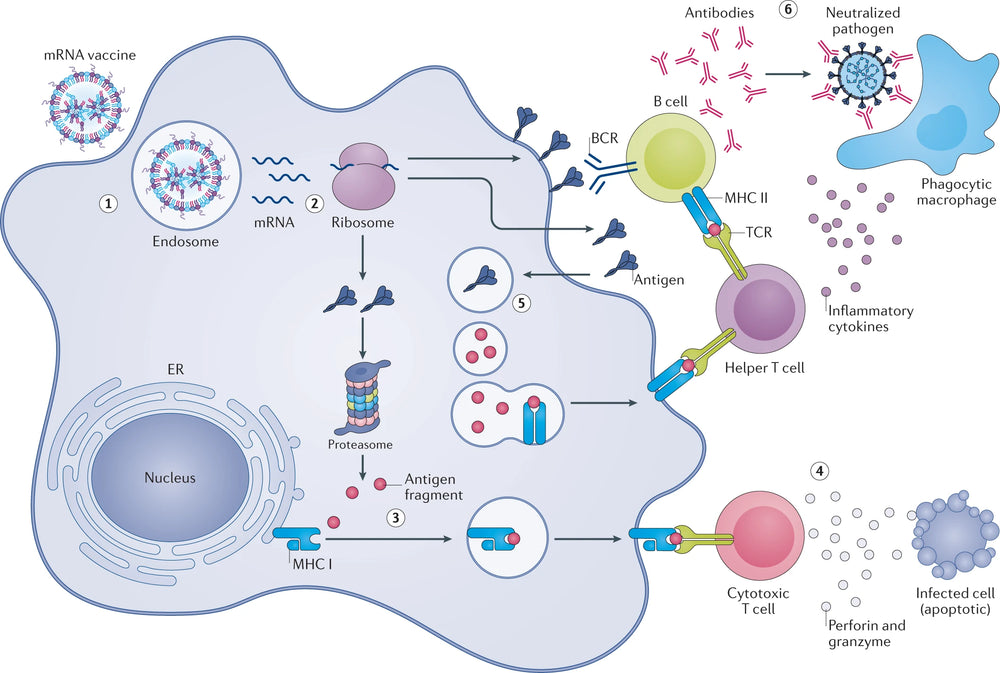
Figure 1. Mechanism of action of mRNA vaccines [1].
The template for mRNA in vitro transcription is typically plasmid DNA, and needs to be linearized before transcription. Linearization ensures the acquisition of mRNA transcripts of definite length and sequence. KACTUS provides restriction endonucleases such as BsaI, BspQI, SapI, XbaI, etc., for the preparation of linearized templates.
Figure 2. (Left) BsaI enzyme activity detection. 1μg of plasmid was added to a 20 μL system, along with 1 μL BsaI (well 1 is undiluted, wells 1-11 are serial 2-fold dilutions), and digested for 30 minutes. The enzyme activity is ≥20 kU/mL. (Right) BsaI star activity detection. 1 μg of plasmid is added to a 20 μL system, along with 1 μL BsaI (well 1 is undiluted; wells 1-11 are serial 2-fold dilutions) and digested for 16 hours. No star activity is observed.
In Vitro Transcription (IVT) is a technique that generates mRNA by mimicking the internal transcription process enzymatically, using linearized plasmid DNA as a template.
Figure 3. The first generation T7 and second generation Premium T7 were used for co-transcriptional capping on different templates to synthesize mRNA and saRNA of different lengths. After purification by lithium chloride precipitation, the dsRNA content were measured. The results show that Premium T7 RNA Polymerase can effectively reduce the production of dsRNA.
Note: MaxPure™ T7 RNA Polymerase is the commercial name for Premium T7 RNA Polymerase (RUO or GMP)
The mRNA produced by IVT has a triphosphate group at the 5’ end, which has strong immunogenicity, necessitating capping and tailing modifications in industrial production. KACTUS offers Vaccinia Capping Enzyme and mRNA Cap 2´-O-Methyltransferase needed for capping modifications, as well as E.coli Poly(A) Polymerase required for tailing modifications. To precisely quantify important indicators such as "capping rate" and "Poly(A) tail product length," we have carefully established an LC-MS platform and developed mature mass spectrometry detection methods to control the release of enzyme products by batch. We can also provide capping rate and tailing determination services according to your needs.
Figure 4. mRNA is capped with Cap1 structure using Vaccinia Capping Enzyme and mRNA Cap 2´-O-Methyltransferase from KACTUS. The capping rate is 95.16% detected via our LC-MS platform. (Note: The capping rate is related to the performance of the enzyme and factors such as the secondary structure of mRNA.)
Linear mRNA obtained from IVT can be further circularized to obtain circRNA. The circularization methods include Type I intron self-splicing, Type II intron self-splicing, T4 RNA Ligase, etc. KACTUS provides T4 RNA Ligase I, T4 RNA Ligase II, and RNase R for in vitro RNA circularization and purification processing of circRNA.
Figure 5. RNase R digests linear RNA (left) without affect circRNA (right).
GMP-Grade: Currently manufactured according to cGMP guidelines.
GMP-Ready: Industrial-Grade enzyme that can be transitioned to our 100,000 sq ft GMP-Grade facility with GMP documentation and testing.
Drug Master Files: Submitted to the FDA Drug Master Files (DMF)



