Focus on Hematoma Target Fms-like Tyrosine Kinase 3 (FLT3)
By Mallory Griffin
About FLT3
Fms-like tyrosine kinase 3 (FLT3) is also known as CD135 (Cluster of differentiation antigen 135) and FLK2 (Fetal liver kinase-2). It is a member of the type III tyrosine receptor family and is a single transmembrane protein consisting of an extracellular domain (including D1-D5) containing five Ig-like domains, a transmembrane region, a proximal membrane domain (JM) and an intracellular tyrosine kinase domain (TKD). Members of the same family also include PDGFRα, PDGFRβ, M-CSFR, KIT. FLT3 is expressed on lineage-restricted myeloid and lymphoid progenitor cells.
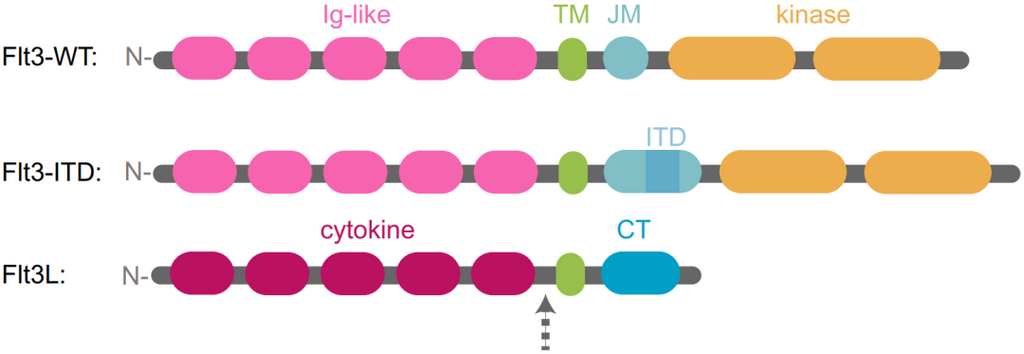

Figure 2: FLT3 binds to FLT3L [2].
FLT3 is activated by binding to its ligand FLT3L, which exists in membrane-bound or soluble form and is produced by bone marrow stromal cells. When FLT3L binds to the D3 domain in FLT3 Ig-like, FLT3 will dimerize, thereby activating downstream signaling pathways, such as PI3K-AKT, JAK-STAT and RAS-MAPK, etc., resulting in the maturation and proliferation of hematopoietic cells. Hence, FLT3 plays an important role in hematological tumor development.

Figure 3: FLT3-FLT3L signaling pathway [3].
FLT3 Targeting Drugs
Small Molecule Inhibitors
FLT3 small-molecule inhibitors have been studied for more than two decades, either inhibiting the ATP-binding pocket in the tyrosine-kinase domain, or inhibiting the inactive conformational epitopes adjacent to the ATP pocket. They can also be used as allosteric regulators. Representative drugs are Gilteritinib, Crenolanib, and FF10101. However, small-molecule compounds have their own limitations, such as low specificity leading to off-target toxicity and single efficacy leading to drug resistance. AML patients treated with FLT3 inhibitors have poor survival within five years.
PROTAC
PROTAC is a technology that integrates the advantages of small molecule compounds and can cause the degradation of target proteins. It has been reported that the research team of Nankai University has developed a highly efficient PROTAC drug for the first-generation FLT3-ITD inhibitor Dovitinib, which has better killing ability against FLT3-ITD-positive AML cells than Dovitinib alone [4].
Antibody Drugs
Monoclonal antibodies targeting FLT3 include IMC-EB10, IMC-NC7, etc., can block the interaction between FL and FLT3 and inhibit the phosphorylation and activation of FLT3. However, monoclonal antibodies alone may not be effective. IMC-EB10 was terminated because the maximum tolerated dose did not reach therapeutic significance. There are other antibody drugs with improved efficacy, specificity, and in vivo kinetics though. These include 4G8SDIEM with optimized Fc segment, FLT3-A192 with protein-peptide coupling, and the double antibody 7370 Anti-FLT3-CD3 that targets both CD3 and FLT3.

Figure 4: 7370 Anti-FLT3-CD3 double antibody targets D4 of FLT3 [5].
T-Cell Therapy
T-cell therapy is a major direction of FLT3 targeted therapy. Autologous T-cell therapy for FLT3-ITD mutant CAR-T cells has been reported to be more effective when combined with FLT3 inhibitors [6],[7]. There is also a dual-target CAR-T developed on this basis. It simultaneously targets FLT3 and FLT3-ITD-related WT-1 antigen to avoid disease recurrence caused by treatment escape. There is also the introduction of cytokines, such as IL-12, into CAR to further improve the efficacy (i.e. AMG 553 in clinical phase I). TCR-T is another strategy. By changing the complementarity-determining region on the TCR that binds to the neoantigen peptide, it can have a higher affinity. Also, the TCR can be transformed into a human-mouse chimera to enhance the stability of the TCR-CD3 complex [8].
There are also recombinant truncated FLT3 ligands and Fc fusion protein drugs developed by Innovent Biologics, which have shown good anti-tumor effects in vivo, and can further exert synergistic effects in combination with PD-1 antibodies. Moreover, small molecular compounds can interfere with the transcription, translation and degradation of FLT3. Finally, compounds such as OTS514 and HSD1169 can inhibit FLT3-related signaling pathways.
KACTUS provides high-quality FLT3 recombinant protein
FLT3 is a mature target for various types of drugs in singular or in combination, thus leaving broad potential for drug development. KACTUS has always paid attention to the field of hematological tumor treatment. With the help of our unique protein expression platform SAMS™, we have successfully developed high-quality recombinant FLT3 and FLT3L proteins of various species and labels, which can be applied to drug discovery, screening, and evaluation. Our quality control standards make them suitable for preclinical CMC research to support the development of relevant drugs. These protein interactions are further validated using technologies such as SPR assay to determine binding kinetics with high sensitivity.
Example Product Data

Figure 5. Immobilized Human FLT3 Ligand, His Tag at 1μg/mL (100μL/Well) on the plate. Dose response curve for Human FLT3, hFc Tag with the EC50 of 30ng/mL determined by ELISA.

Figure 6. Mouse FLT3 Ligand, hFc Tag captured on CM5 Chip via Protein A can bind Human FLT3, His Tag with an affinity constant of 13.81 nM as determined in SPR assay (Biacore T200).

Figure 7. Human FLT3 Ligand, hFc Tag immobilized on CM5 Chip can bind Mouse FLT3, hFc Tag with an affinity constant of 35.07 nM as determined in SPR assay (Biacore T200).
KACTUS Products
|
|
Mouse FLT3 Ligand, His Tag |
References
[1] Wilson KR, Villadangos JA, Mintern JD. Dendritic cell Flt3 - regulation, roles and repercussions for immunotherapy. Immunol Cell Biol. 2021 Oct;99(9):962-971. doi: 10.1111/imcb.12484. Epub 2021 Jul 2. PMID: 34097779.
[2] Roskoski R Jr. The role of small molecule Flt3 receptor protein-tyrosine kinase inhibitors in the treatment of Flt3-positive acute myelogenous leukemias. Pharmacol Res. 2020 May;155:104725. doi: 10.1016/j.phrs.2020.104725. Epub 2020 Feb 25. PMID: 32109580.
[3] Acharya B, Saha D, Armstrong D, Lakkaniga NR, Frett B. FLT3 inhibitors for acute myeloid leukemia: successes, defeats, and emerging paradigms. RSC Med Chem. 2022 May 23;13(7):798-816. doi: 10.1039/d2md00067a. PMID: 35923716; PMCID: PMC9298189.
[4] Cao S, Ma L, Liu Y, Wei M, Yao Y, Li C, Wang R, Liu N, Dong Z, Li X, Li M, Wang X, Yang C, Yang G. Proteolysis-Targeting Chimera (PROTAC) Modification of Dovitinib Enhances the Antiproliferative Effect against FLT3-ITD-Positive Acute Myeloid Leukemia Cells. J Med Chem. 2021 Nov 25;64(22):16497-16511. doi: 10.1021/acs.jmedchem.1c00996. Epub 2021 Oct 25. PMID: 34694800.
[5] Yeung YA, Krishnamoorthy V, Dettling D, Sommer C, Poulsen K, Ni I, Pham A, Chen W, Liao-Chan S, Lindquist K, Chin SM, Chunyk AG, Hu W, Sasu B, Chaparro-Riggers J, Djuretic I. An Optimized Full-Length FLT3/CD3 Bispecific Antibody Demonstrates Potent Anti-leukemia Activity and Reversible Hematological Toxicity. Mol Ther. 2020 Mar 4;28(3):889-900. doi: 10.1016/j.ymthe.2019.12.014. Epub 2020 Jan 14. PMID: 31981494; PMCID: PMC7054815.
[6] Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, Bonig H, Herr W, Sierra J, Einsele H, Hudecek M. CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018 May;32(5):1168-1179. doi: 10.1038/s41375-018-0009-0. Epub 2018 Feb 5. PMID: 29472720.
[7] Li KX, Wu HY, Pan WY, Guo MQ, Qiu DZ, He YJ, Li YH, Yang DH, Huang YX. A novel approach for relapsed/refractory FLT3mut+ acute myeloid leukaemia: synergistic effect of the combination of bispecific FLT3scFv/NKG2D-CAR T cells and gilteritinib. Mol Cancer. 2022 Mar 4;21(1):66. doi: 10.1186/s12943-022-01541-9. Erratum in: Mol Cancer. 2022 Jun 23;21(1):134. PMID: 35246156; PMCID: PMC8896098.
[8] Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006 Sep 1;66(17):8878-86. doi: 10.1158/0008-5472.CAN-06-1450. PMID: 16951205; PMCID: PMC2147082.