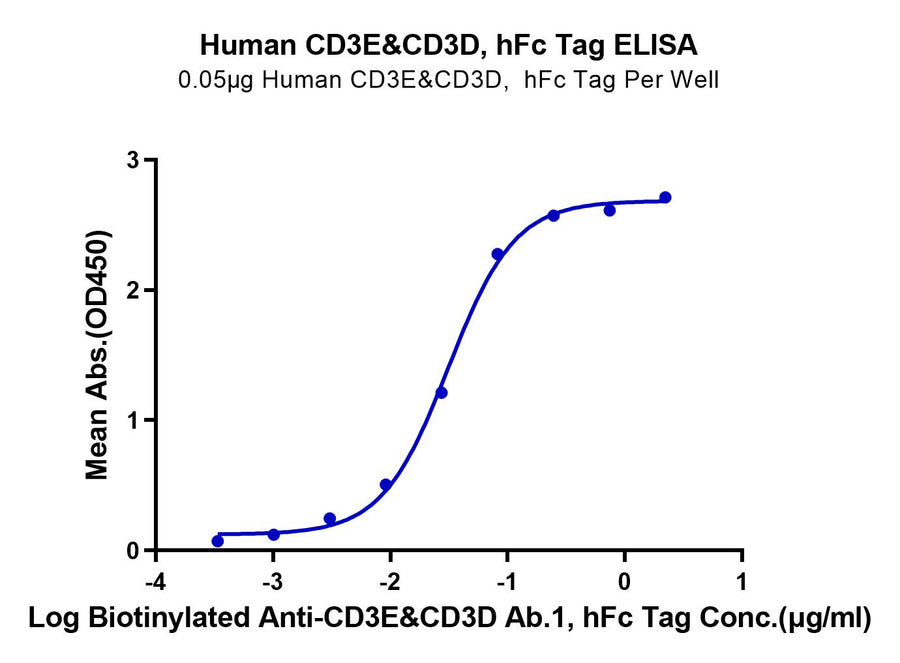
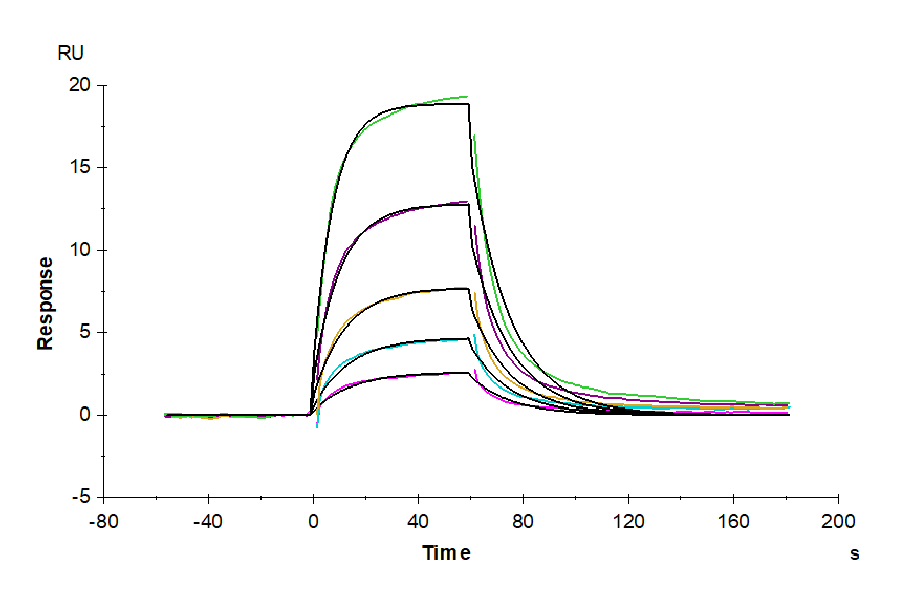
Product Description |
Recombinant Human VSIG8 Protein is expressed from HEK293 with His tag at the C-Terminus.It contains Val22-Gly263. |
Product Background |
VSIG8 (V-Set And Immunoglobulin Domain Containing 8) is a Protein Coding gene. Diseases associated with VSIG8 include Retinitis Pigmentosa 30 and Monilethrix. An important paralog of this gene is CXADR.The present work helps characterize the component V-set and immunoglobulin domain containing 8 (VSIG8) in hair shaft and nail plate to assist in understanding its possible relation to disease states. |
Product Category |
Recombinant Protein / Antibody discovery |
Protein |
VSIG8 |
Synonyms |
VSIG8; C1orf204 |
Accession |
|
Species |
Human |
Biotinylated |
no |
Amino Acid Range |
Val22-Gly263 |
Molecular Weight |
The protein has a predicted MW of 28.2 kDa. Due to glycosylation, the protein migrates to 29-32 kDa based on Bis-Tris PAGE result. |
Product Tag |
C-His |
Expression System |
HEK293 |
Purity |
> 95% as determined by Bis-Tris PAGE |
Endotoxin |
Less than 1EU per ug by the LAL method. |
Form |
Lyophilized |
Shipping |
Shipped at ambient temperature. |
Formulation |
Lyophilized from 0.22um filtered solution in 20mM NaAc,150mM NaCl (pH 4.0). Normally 8% trehalose is added as protectant before lyophilization. |
Reconstitution |
Centrifuge the tube before opening. Reconstituting to a concentration more than 100 ug/ml is recommended. Dissolve the lyophilized protein in 20mM NaAc,150mM NaCl (pH 4.0). |
Stability And Storage |
-20 to -80°C for 12 months as supplied from date of receipt.;-80°C for 3 months after reconstitution.;Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
Related Products
Have more questions?
Check out our Frequently Asked Questions page for more details about product specifications, ordering, and shipments.
Contact Us
More from Antibody Drug Discovery Proteins
Recently viewed