- Express System: HEK293
- Product Tag: C-His
- Purity: > 95% as determined by Bis-Tris PAGE;> 95% as determined by HPLC
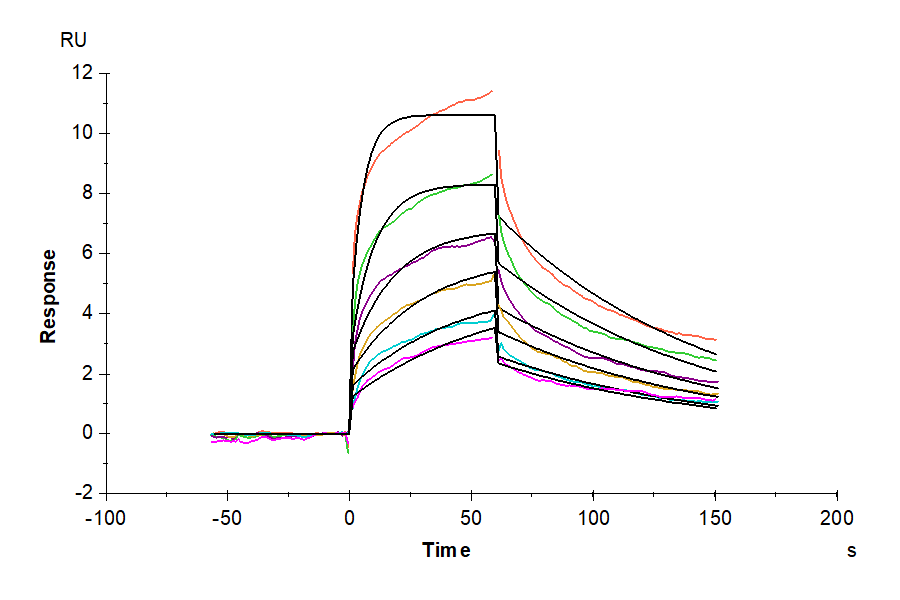
- Activity: The affinity constant of 0.12 uM as determined in SPR assay (Biacore T200).
- Amino Acid Range: Gln29-Ala248
-
Other Available Proteins:
Loading...








