Cell and Antibody Therapies Targeting HLA-G
By Mallory Griffin
On December 19, 2022, the FDA approved the Phase I/IIa clinical trial application of IVS-3001, an anti-HLA-G Chimeric Antigen Receptor-T Cell (CAR-T) cell therapy developed by Invectys and CTMC (a joint venture between MD Anderson Cancer Center and National Resilience, Inc.). The study will evaluate the safety, tolerability, and pharmacokinetics of IVS-3001 in patients with locally advanced HLA-G-positive solid tumors.

Figure 1. Mechanism of action of IVS-3001 [1].
HLA-G (Human Leukocyte Antigen G) is a non-classical major histocompatibility complex MHC Ib protein, which is an important immune tolerance molecule in the body. It is expressed in embryonic trophoblast cells and can resist the maternal immune system. This is vital to the maintenance of normal embryonic development. However, this protective mechanism is also utilized by tumor cells. HLA-G is expressed on the surface of a variety of tumor cells and acts as an immune checkpoint, which can interact with the inhibitory receptors LILRB1 (ILT2) and LILRB2 (ILT4) on the surface of immune cells. HLA-G and LILRB1 interact to suppress T cell function and protect cells from direct cytotoxicity by CD8+ cells. Conversely, the interaction of HLA-G and LILRB2 inhibits the maturation of myeloid cells and converts them into suppressive antigen-presenting cells, thereby establishing an immunosuppressive tumor microenvironment. Thus, HLA-G directly and indirectly assists tumors in immune evasion.

Figure 2. HLA-G suppresses the immune response [1].
HLA-G is not expressed in non-pregnant normal adult tissues. Aside from fetal tissue, HLA-G is a tumor-specific antigen, and is actually highly expressed in a variety of malignant tumor tissues. Thus, it may have fewer off-target effects and better efficacy. It is a new tumor marker suitable for cancer diagnosis, treatment, or prognosis guidance. In addition, the expression pattern of HLA-G is different from that of general immune checkpoints such as PD-1, which makes it eligible as a candidate for tumors non-response to PD-1 therapy or for synergistic immune therapies. Treatments targeting HLA-G have great potential.

Figure 3. Signaling pathway mediated by HLA-G and its receptor [2].
Tizona Therapeutics, Invectys and Janssen are currently developing HLA-G antibodies and CAR-T drugs. The FIC antibody TTX-080, developed by Tizona, can block the HLA-G-LILRB1 pathway and activate a variety of immune cells. It is the first HLA-G antibody drug and is currently in phase I clinical treatment for solid tumors [3]. In addition, Janssen’s double antibody JNJ-78306358 targeting both CD3 and HLA-G is undergoing phase I clinical trials for solid tumors.
High-quality recombinant HLA-G proteins to help the development of drugs targeting HLA-G
Product Advantages
- Monomers & tetramers
- Biotinylation
- Fluorescent labeling
- Human, rhesus macaque, cynomolgus, mouse
- High purity
- Proven biological activity
HLA-G Proteins Product List
|
Biotinylated Human HLA-G Tetramer |
|
|
|
Product Validation Data
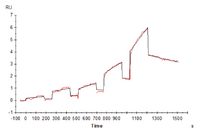
Figure 4. Human LILRB2, hFc Tag captured on CM5 Chip via Protein A can bind Human HLA-G Tetramer with an affinity constant of 4.62 nM as determined in SPR assay (Biacore T200).
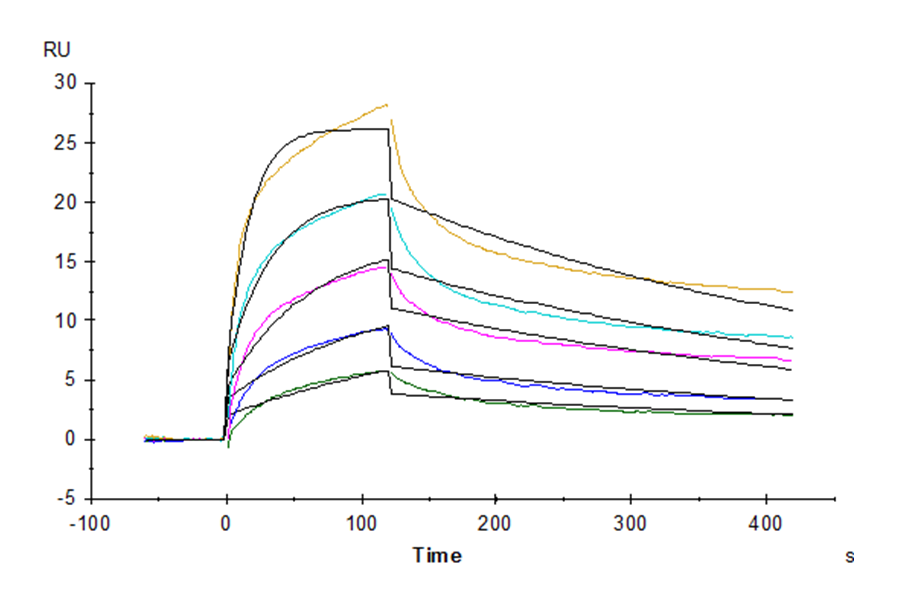
Figure 5. Rhesus macaque LILRB1, hFc Tag captured on CM5 Chip via Protein A can bind Rhesus macaque HLA-G Tetramer, His Tag with an affinity constant of 1.74 nM as determined in SPR assay (Biacore T200).
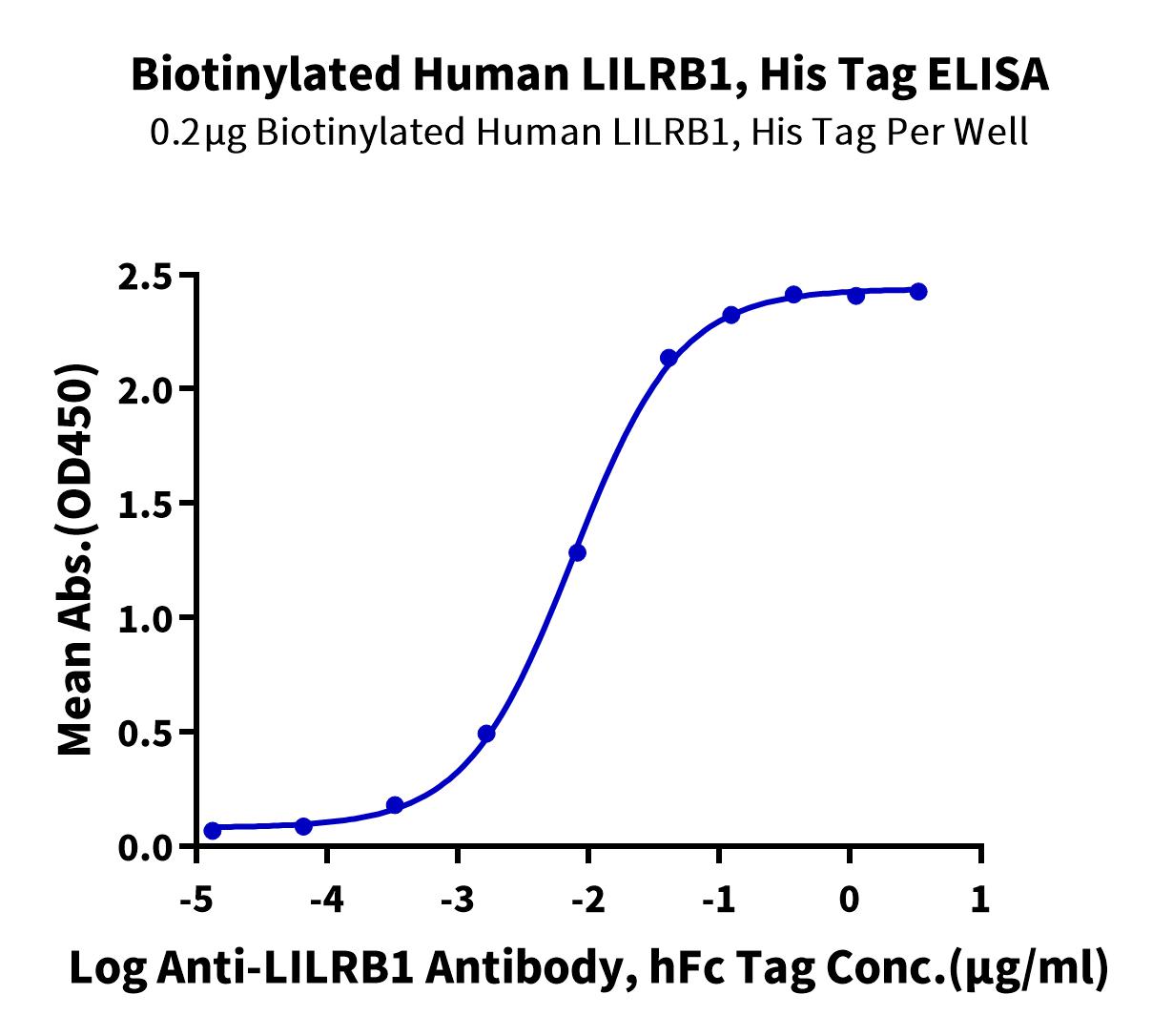
Figure 6. Immobilized Biotinylated Human LILRB1, His Tag at 2ug/ml (100ul/well) on the streptavidin precoated plate (5ug/ml). Dose response curve for Anti-LILRB1 Antibody, hFc Tag with the EC50 of 7.5ng/ml determined by ELISA.
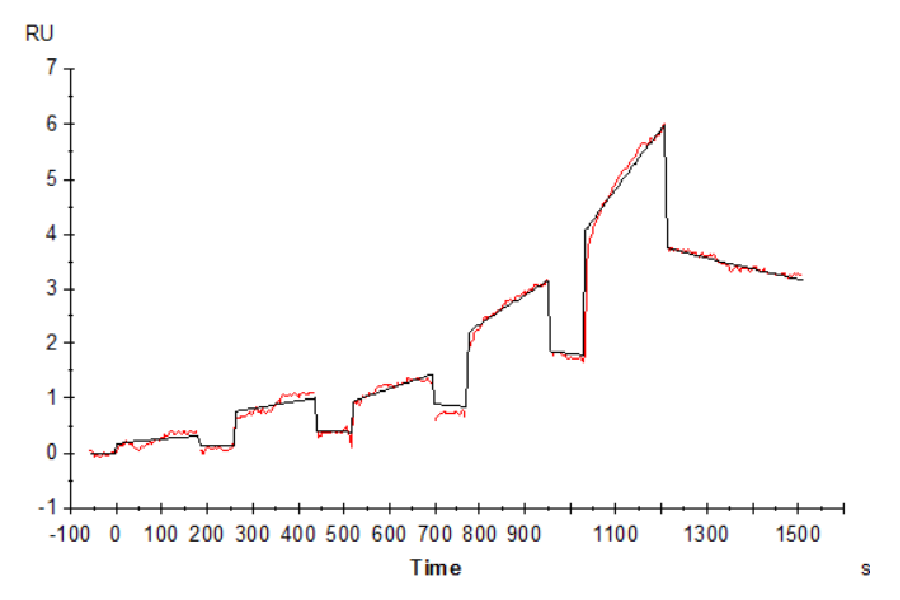
Figure 7. Cynomolgus LILRB2, His Tag immobilized on CM5 Chip can bind Cynomolgus HLA-G Complex Tetramer, His Tag with an affinity constant of 852 nM as determined in SPR assay (Biacore T200).
References
[1] https://www.invectys.com/products-pipeline/hla-g-platform/
[2] Nguyen-Lefebvre AT, Ajith A, Portik-Dobos V, Horuzsko DD, Mulloy LL, Horuzsko A. Mouse models for studies of HLA-G functions in basic science and pre-clinical research. Hum Immunol. 2016 Sep;77(9):711-9.
[3] Gilead Buys into Tizona's Anti-HLA-G Strategy. Cancer Discov. 2020 Oct;10(10):1433.