Biotinylated Mouse Siglec-15/CD33L3 Protein (Primary Amine Labeling) (SIG-MM115B) Summary
Product Description |
Recombinant Biotinylated Mouse Siglec-15/CD33L3 Protein (Primary Amine Labeling) is expressed from HEK293 with His tag at the C-Terminus.It contains Arg24-Thr262. |
Product Background |
Siglec-15 is a transmembrane glycoprotein in the Siglec family of sialic acid-binding immune regulatory molecules. Mature human Siglec-15 consists of a 244 amino acid extracellular domain (ECD) with two Ig-like domains, a 21 aa transmembrane segment, and a 44 aa cytoplasmic domain. Siglec-15 is a potential therapeutic target for osteoporosis and plays a conserved regulatory role in the immune system of vertebrates. |
Product Category |
Recombinant Protein / Antibody discovery / Antibody discovery | Others |
Biotinylated Mouse Siglec-15/CD33L3 Protein (Primary Amine Labeling) (SIG-MM115B) Specifications/Details
Protein |
Siglec-15/CD33L3 |
Synonyms |
CD33 molecule-like 3; CD33L3; HsT1361; Siglec15; CD33 antigen-like 3; SIGLEC-15 |
Accession |
|
Species |
Mouse |
Biotinylated |
yes |
Amino Acid Range |
Arg24-Thr262 |
Molecular Weight |
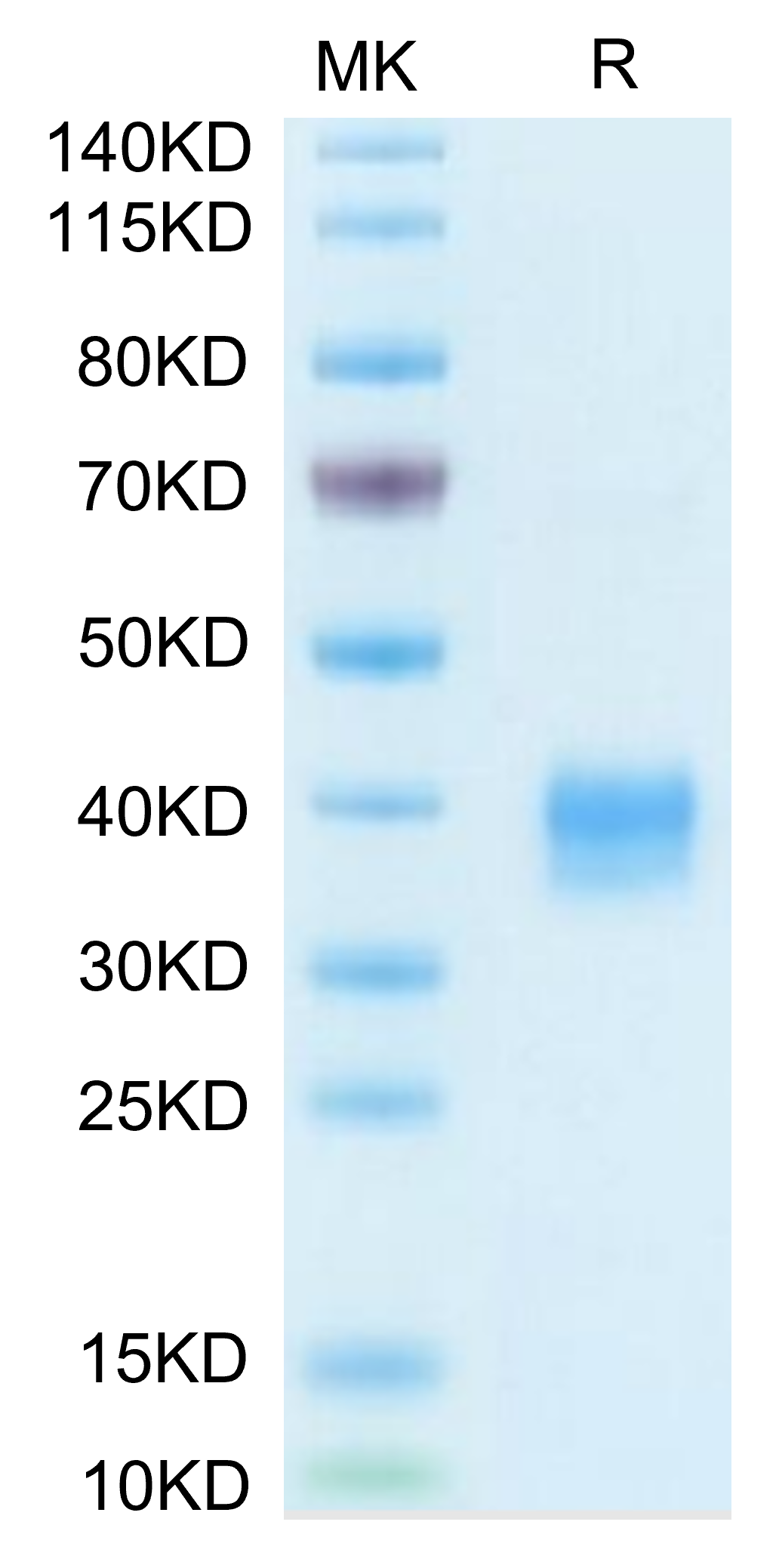
The protein has a predicted MW of 26.7 kDa. Due to glycosylation, the protein migrates to 35-43 kDa based on Bis-Tris PAGE result. |
Product Tag |
C-His |
Expression System |
HEK293 |
Purity |
> 95% as determined by Bis-Tris PAGE |
Endotoxin |
Less than 1 EU per ug by the LAL method. |
Form |
Liquid |
Shipping |
Shipped with dry ice. |
Formulation |
Supplied as 0.22um filtered solution in 20mM PB, 0.5M NaCl, 0.1M L-arginine (pH 7.4). |
Stability And Storage |
Valid for 12 months from date of receipt when stored at -80°C.; Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
Related Products
Have more questions?
Check out our Frequently Asked Questions page for more details about product specifications, ordering, and shipments.
Contact Us
More from Antibody Drug Discovery Proteins
Recently viewed