Cynomolgus GDF15 Protein (GDF-CE115) Summary
Product Description |
Recombinant Cynomolgus GDF15 Protein is expressed from E.coli with His tag at the N-Terminus.It contains Arg193-Val308. |
Product Background |
Growth and differentiation factor 15 (GDF15) is an inflammation-associated hormone with poorly defined biology. Here, we investigated the role of GDF15 in bacterial and viral infections. Inflammation induced GDF15, and that GDF15 was necessary for surviving both bacterial and viral infections, as well as sepsis. The protective effects of GDF15 were largely independent of pathogen control or the magnitude of inflammatory response, suggesting a role in disease tolerance. |
Product Category |
Recombinant Protein / Antibody discovery / Antibody discovery | Others |
Cynomolgus GDF15 Protein (GDF-CE115) Specifications/Details
Protein |
GDF15 |
Synonyms |
GDF-15; MIC-1; NAG-1; PDF; PLAB; PTGFB; GDF15; MIC1; RG-1; Placental TGF-beta; PTGF-beta; PTGFBPTGF-beta; Placental TGF-β; PTGF-β; PTGFBPTGF-β |
Accession |
|
Species |
Cynomolgus |
Biotinylated |
no |
Amino Acid Range |
Arg193-Val308 |
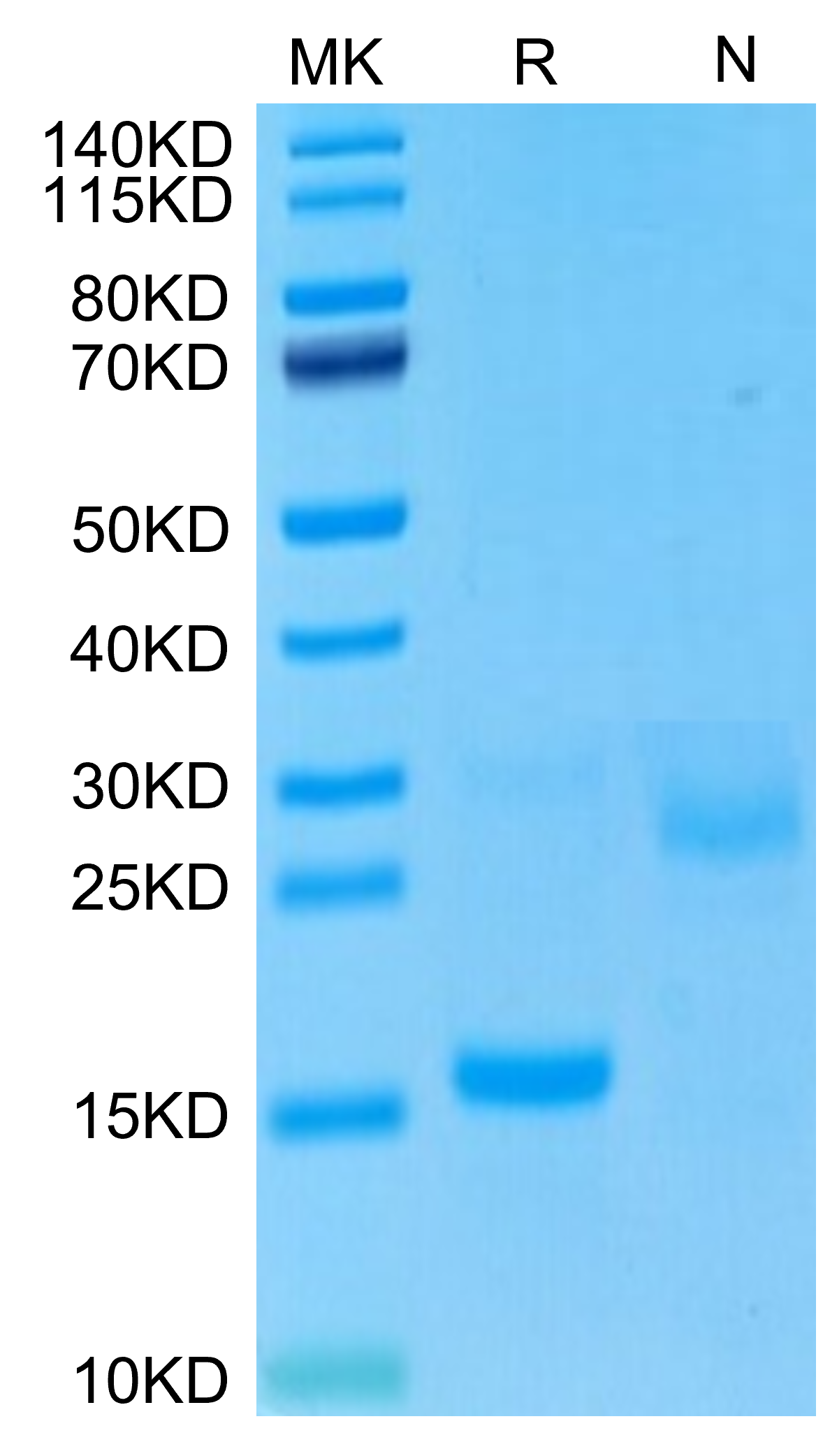
Molecular Weight |
The protein has a predicted MW of 14.15 kDa. The protein migrates to 15-18 kDa based on Bis-Tris PAGE result. |
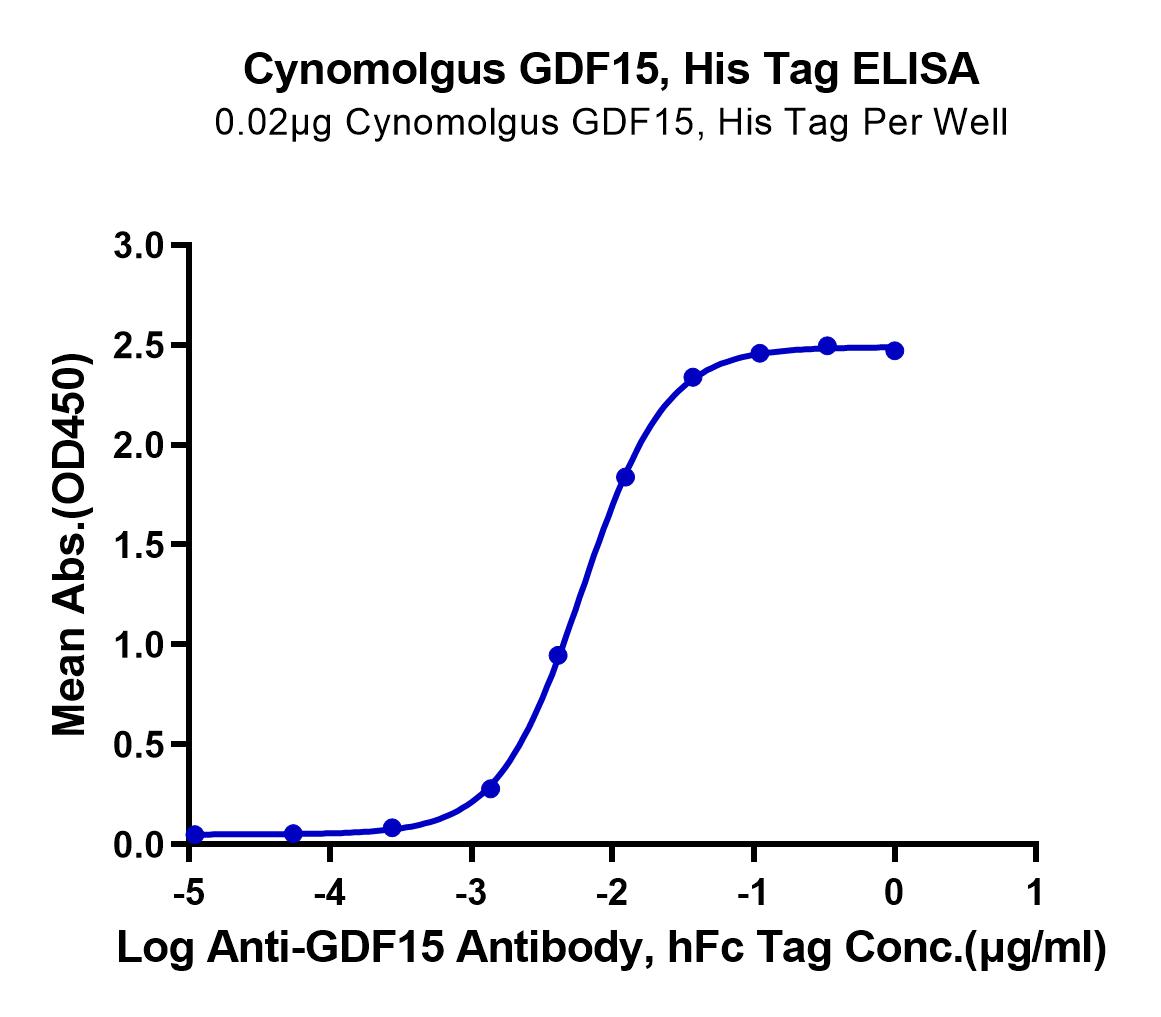
Activity |
Immobilized Cynomolgus GDF15, His Tag at 5 ug/ml (100 ul/well) on the plate. Dose response curve for Biotinylated Cynomolgus GFRAL, His Tag with the EC50 of 39.9 ng/ml determined by ELISA (QC Test). |
Product Tag |
N-His |
Expression System |
E.coli |
Purity |
> 95% as determined by Bis-Tris PAGE |
Endotoxin |
Less than 1 EU per ug by the LAL method. |
Shipping, Reconstitution, & Storage
Form |
Lyophilized |
Shipping |
Shipped at ambient temperature. |
Formulation |
Lyophilized from 0.22 um filtered solution in 50mM HAc (pH 2.9). Normally 8% trehalose is added as protectant before lyophilization. |
Reconstitution |
Dissolve the lyophilized protein in 50mM HAc (pH 2.9). It is recommended to resuspend at 0.5 mg/mL if the lyophilized powder is 100 ug or less, at 1mg/ml for 500ug or 1mg lyophilized powder. Do not mix by vortex or vigorous shaking. It is recommended to resuspend at 0.5 mg/mL if the lyophilized powder is 100 ug or less, at 1mg/ml for 500ug or 1mg lyophilized powder. Do not mix by vortex or vigorous shaking. |
Stability And Storage |
-20 to -80°C for 12 months as supplied from date of receipt.;-80°C for 3 months after reconstitution.;Recommend to aliquot the protein into smaller quantities for optimal storage. Please minimize freeze-thaw cycles. |
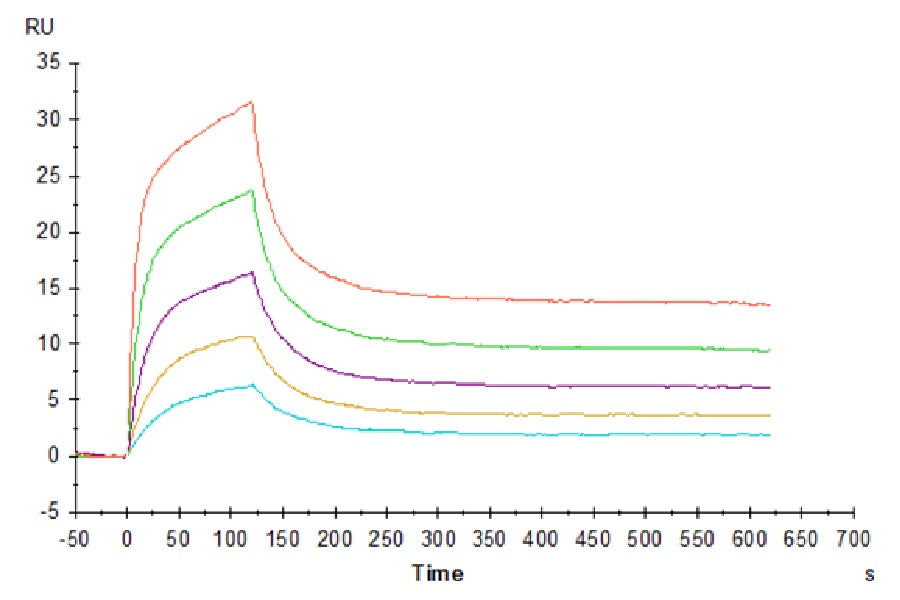
Validation Images
Related Products
Have more questions?
Check out our Frequently Asked Questions page for more details about product specifications, ordering, and shipments.
Contact Us
Recently viewed