CDH17: A Safe CAR-T Target for Treatment of Solid Tumors
By Mallory Griffin
Chimeric antigen receptor T cell (CAR-T) therapy has been successful in the field of hematological malignancies, but it is not effective in solid tumors. However, this situation may be about to change. A VHH1-CAR T cell targeting Cadherin 17 (CDH17) was developed by researchers at the University of Pennsylvania School of Medicine, which specifically eradicated CDH17-expressing neuroendocrine tumors (NET) and gastric, pancreatic, and colorectal cancer cells without damaging CDH17-expressing normal cells. This indicates that CDH17 is a potentially safer CAR-T therapeutic target. The results of this study were published in the journal Nature Cancer on March 21, 2022 [1].
About Cadherin 17 Protein (CDH17)
CDH17, also known as liver–intestine cadherin or LI-cadherin, is a member of the 7-D subfamily of the cadherin family and is mainly expressed in the gastrointestinal system. Compared with other common CAR-T targets, CDH17 has tissue specificity so the corresponding CAR-T drug toxicity is relatively low. CDH17 has a longer extracellular region, containing seven cadherin domains, and a shorter intracellular region (Figure 1). Studies have shown that CDH17 is highly expressed in well-differentiated advanced tumors, but rarely expressed in poorly differentiated tumors [2]. It is often used clinically as an immunohistochemical marker for the diagnosis of digestive system adenocarcinoma.

Figure 1. CDH17 structure [3]
CDH17 is a Ca2+-dependent cell adhesion molecule that can activate cell adhesion signals after binding to integrin molecules such as integrin α2β1 (Figure 2). Due to the short intracellular region, CDH17 cannot mediate tight junctions by interacting with intracellular cadherins and actin, but rather by directly binding intracellular scaffold proteins. Due to localized expression at the tight junctions between cells, macromolecular targeted drugs may not be able to reach or bind CDH17. On the contrary, tumor cells express CDH17 on all cell surfaces due to lack of polarity, so this may also be one of the reasons why the VHH1-CAR T cells mentioned above only attack tumor cells but not normal cells.

Figure 2. CDH17 binds Integrin α2β1 [4]
Cadherin 17 as a Therapeutic Target
As a potential safety target, CDH17 is promising in the treatment of solid tumors. At present, the fastest-growing CDH17-targeted drug is Boehringer Ingelheim's dual-antibody drug, BI 905711, which can bind to both CDH17 and the death receptor TRAILR2 on the surface of tumor cells, inducing tumor cell apoptosis without damaging CDH17-negative normal tissue cells (Figure 3).

Figure 3. The mechanism of action of BI 905711 [5]
Other dual-antibody drugs include ARB202 developed by Hong Kong Aibel Pharmaceutical Technology Co., Ltd., which targets the RGD domain of CD3 and CDH7 respectively, prevents CDH17 from binding to integrin, and recruits T cells for tumor killing. The treatment of ARB202 for patients with solid tumors such as colorectal cancer, cholangiocarcinoma, pancreatic cancer, and gastric cancer is in Phase I clinicals. In addition, the cell therapy CHM 2101 (CDH17 CAR-T) and CHM2301 (CDH17 CAR-NK) deployed by Chimeric Therapeutics are both in the preclinical development stage. The drug market layout of CDH17 is quite broad, and it is expected that there will be good progress in the next period of time.
|
Drug Name |
Type of Drug |
Target |
Development Agency |
Stage |
Indications |
|
BI 905711 |
Dual Antibody |
CDH17. TRAILR2 |
Boehringer Ingelheim |
Phase I |
Cholangiocarcinoma, colorectal, stomach, esophageal, & pancreatic cancer |
|
ARB202 |
Dual Antibody |
CDH17, CD3 |
Arbele |
Phase I |
Cholangiocarcinoma, liver, colorectal, pancreatic, & stomach cancer |
|
CDH17 A4 |
Monoclonal Antibody |
CDH17 |
Oxford Biotherapeutics |
Preclinical |
/ |
|
CHM 2101 |
CAR-T |
CDH17 |
Chimeric Therapeutics; University of Pennsylvania |
Preclinical |
Neuroendocrine tumor, nodules, colon, esophageal, & stomach cancer |
|
CHM 2301 |
CAR-NK |
CDH17 |
Chimeric Therapeutics |
Preclinical |
Solid tumor |
Overcoming solid tumors has always been a direction of human efforts. CDH17 may represent a new and safer drug development target, which has implications for future cell therapy, especially CAR cell therapy.
Quality Cadherin 17 Proteins From KACTUS
KACTUS has independently designed and developed a line of highly active CDH17 recombinant proteins, covering a variety of species and tags. These can meet customers' needs in animal immunization, antibody screening, and epitope identification in different scenarios. All products have passed strict quality monitoring to ensure the batch-to-batch stability of the products. KACTUS is helping to progress the development of CDH17-targeted drugs through high-quality CDH17 products.
Product Validation Data

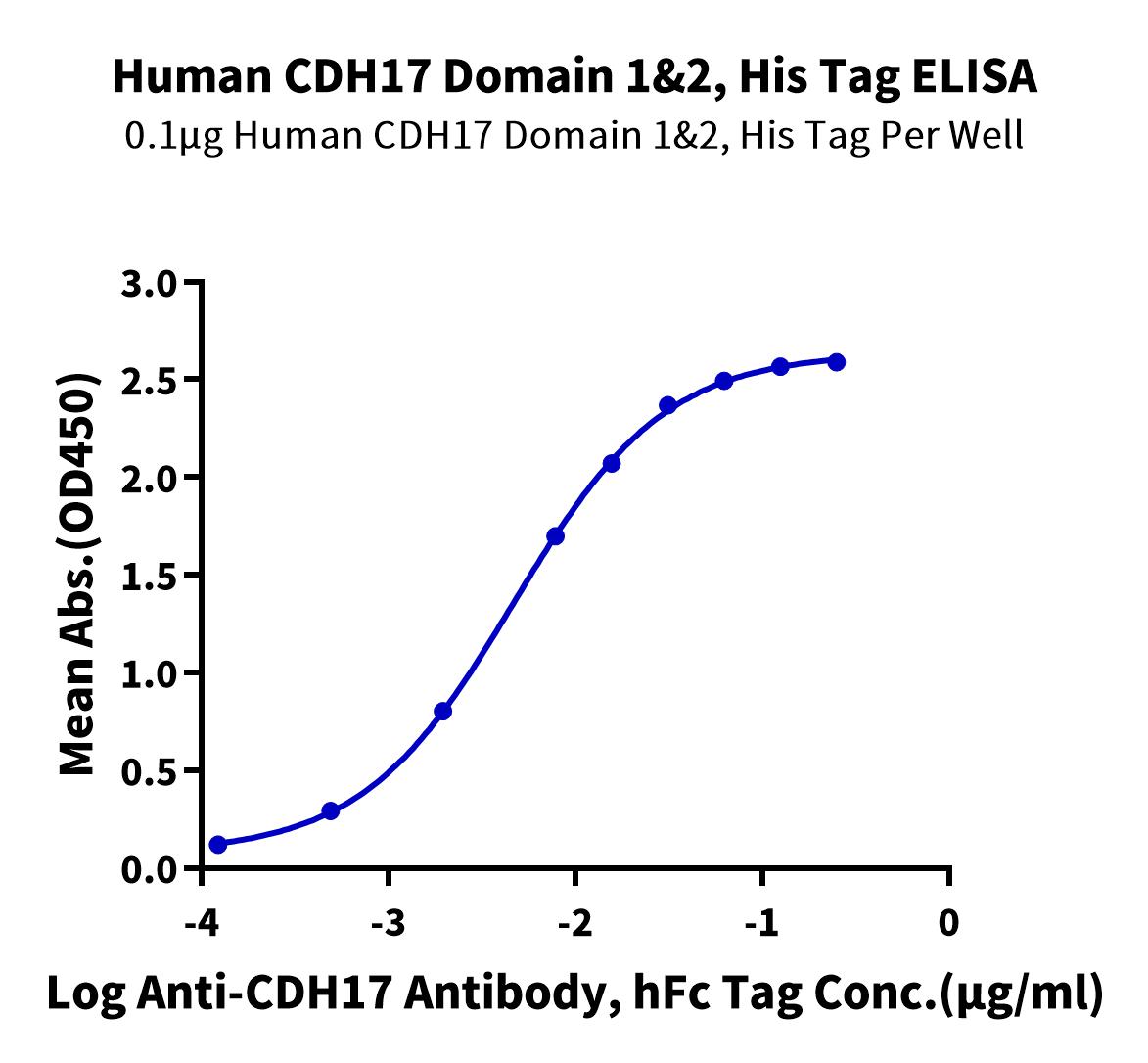
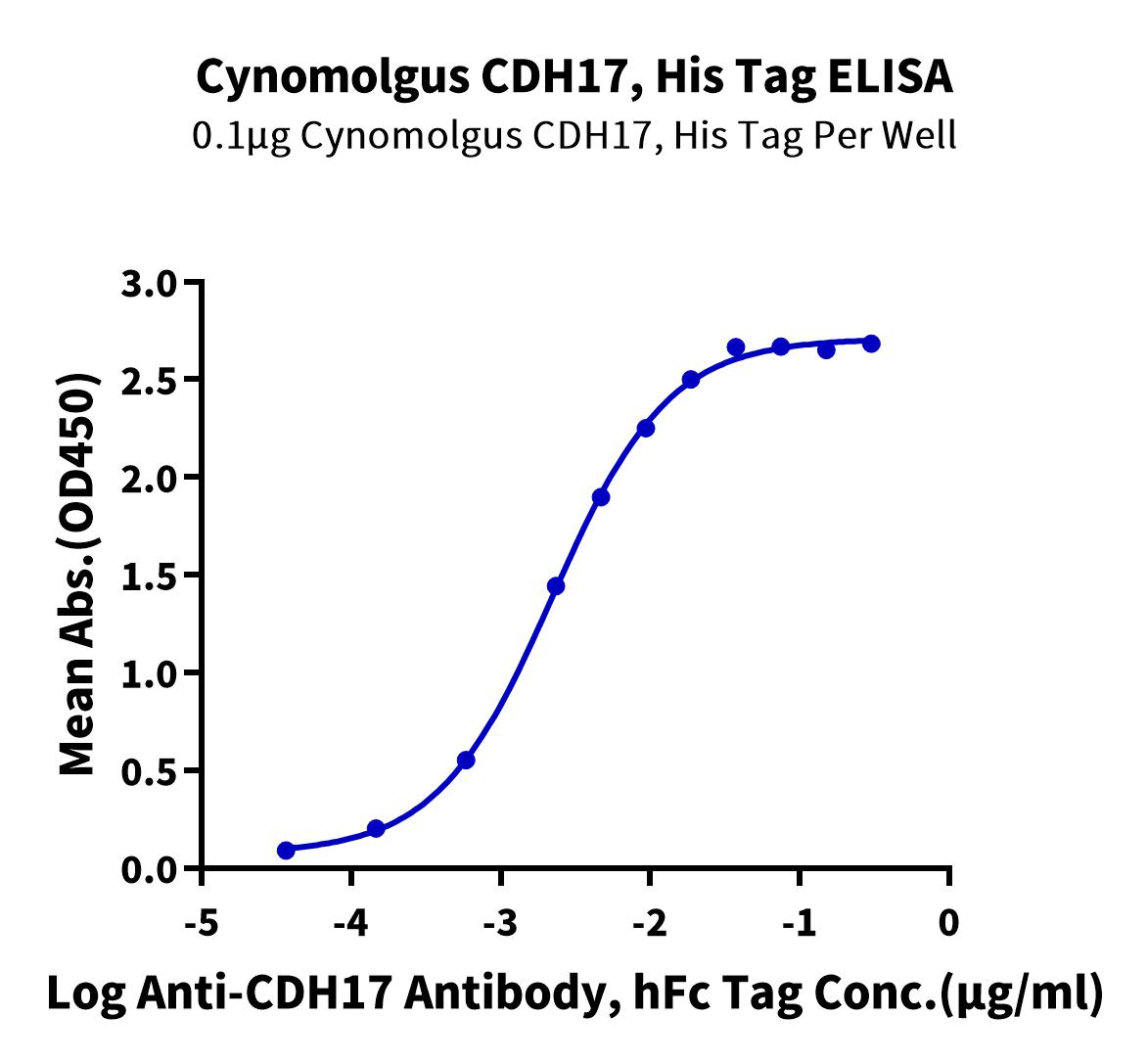
Cadherin 17 Product List
KACTUS offers a catalog portfolio of high-quality Cadherin 17 proteins with high purity and bioactivity.
|
Catalog # |
Product Description |
|
Human CDH17, His Tag |
|
| CDH-HM117B | Biotinylated Human CDH17, His-Avi Tag |
| CDH-HM217 | Human CDH17, hFc Tag |
| CDH-HM1D5 | Human CDH17 Domain 1&2, His Tag |
| CDH-HM3D3 | Human CDH17 Domain 3&4, mFc Tag |
| CDH-HM1D4 | Human CDH17 Domain 5-7, His Tag |
| CDH-HM1D4B | Biotinylated Human CDH17 Domain 5-7, His Tag |
| CDH-HM1D1 | Human CDH17 Domain 1-6, His Tag |
|
FITC-Labeled Human CDH17/Cadherin 17 Protein |
|
|
Mouse CDH17, His Tag |
|
|
Cynomolgus CDH17, His Tag |
|
| CDH-RM117 |
Rhesus macaque CDH17, His Tag
|
|
Human ITGA2&ITGB1, His Tag |
|
|
Mouse ITGA2&ITGB1, His Tag |
References
[1] Feng Z, He X, Zhang X, Wu Y, Xing B, Knowles A, Shan Q, Miller S, Hojnacki T, Ma J, Katona BW, Gade TPF, Schrader J, Metz DC, June CH, Hua X. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat Cancer. 2022 May;3(5):581-594.
[2] Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology. 2002 Nov;123(5):1565-77.
[3] Caporuscio C, Holmes D, Olah TV, Shipkova P. Immunoaffinity enrichment LC-MS/MS quantitation of CDH17 in tissues. Bioanalysis. 2020 Oct;12(20):1439-1447.
[4] Bartolomé RA, Peláez-García A, Gomez I, Torres S, Fernandez-Aceñero MJ, Escudero-Paniagua B, Imbaud JI, Casal JI. An RGD motif present in cadherin 17 induces integrin activation and tumor growth. J Biol Chem. 2014 Dec 12;289(50):34801-14.
[5] https://pro.boehringer-ingelheim.com/inoncology/














